Abstract
Purpose
Glucagon like peptide-1 (7-36) amide (GLP-1) is an incretin hormone with multiple salutary cardiovascular effects. A short course of the GLP-1 analogue Exendin-4 (Ex-4) in the neonatal period prevents the development of mitochondrial dysfunction and oxidative stress in a rat prone to obesity and diabetes. We sought to evaluate whether neonatal Ex-4 can exert the same effect in the normal rat heart, as well as whether Ex-4 could affect susceptibility to cardiac reperfusion injury.
Methods
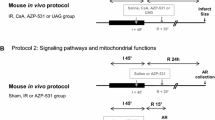
After birth, Sprague Dawley rat pups were given either Ex-4 (1 nmole/kg body weight) or vehicle (1% BSA in 0.9% saline) subcutaneously for 6 days. Animals were studied at juvenile (4–6 weeks) and adult (8–9 months) ages. Using the Langendorff isolated perfused heart, cardiovascular function was assessed at baseline and following ischemia-reperfusion. Mitochondria were isolated from fresh heart tissue, and oxidative phosphorylation and calcium sequestration were analyzed. TBARS, MnSOD activity, and non-enzymatic anti-oxidant capacity were measured to assess the degree of oxidative stress present in the two groups.
Results
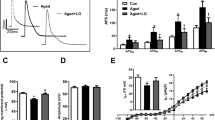
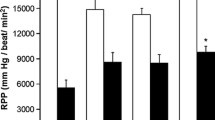
Both at the juvenile and adult age, Ex-4 treated rats demonstrated improved recovery from an ischemic insult. Rates of oxidative phosphorylation were globally reduced in adult, but not juvenile Ex-4 treated animals. Furthermore, mitochondria isolated from adult Ex-4 treated rats sequestered less calcium before undergoing the mitochondrial permeability transition. Oxidative stress did not differ between groups at any time point.
Conclusion
A short course of Exendin-4 in the neonatal period leads to protection from ischemic injury and a preconditioned mitochondrial phenotype in the adult rat.

Similar content being viewed by others
References
Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9.
Nikolaidis LA, Elahi D, Hentosz T, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–61.
Nikolaidis LA, Mankad S, Sokos GG, et al. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5.
Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–51.
Nikolaidis LA, Doverspike A, Hentosz T, et al. Glucagon-like peptide-1 limits myocardial stunning following brief coronary occlusion and reperfusion in conscious canines. J Pharmacol Exp Ther. 2005;312:303–8.
Bose AK, Mocanu MM, Carr RD, Yellon DM. Glucagon like peptide-1 is protective against myocardial ischemia/reperfusion injury when given either as a preconditioning mimetic or at reperfusion in an isolated rat heart model. Cardiovasc Drugs Ther. 2005;19:9–11.
Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83.
Zhao T, Parikh P, Bhashyam S, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317:1106–13.
Halestrap AP, Clarke SJ, Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–31.
Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52:734–40.
Raab EL, Vuguin PM, Stoffers DA, Simmons RA. Neonatal exendin-4 treatment reduces oxidative stress and prevents hepatic insulin resistance in intrauterine growth-retarded rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1785–94.
McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969;244:6049–55.
Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–90.
Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–47.
Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–7.
Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Reversible blockade of electron transport during ischemia protects mitochondria and decreases myocardial injury following reperfusion. J Pharmacol Exp Ther. 2006;319:1405–12.
Ockaili RA, Bhargava P, Kukreja RC. Chemical preconditioning with 3-nitropropionic acid in hearts: role of mitochondrial K(ATP) channel. Am J Physiol Heart Circ Physiol. 2001;280:H2406–11.
Shiva S, Gladwin MT. Nitrite mediates cytoprotection after ischemia/reperfusion by modulating mitochondrial function. Basic Res Cardiol. 2009;104:113–9.
Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–49.
Ajamieh HH, Candelario-Jalil E, Fernandez OS, Gerbes AL. Ischaemic and pharmacological preconditionings protect liver via adenosine and redox status following hepatic ischaemia-reperfusion in rats. Clin Sci (Lond). 2008.
Asimakis GK, Lick S, Patterson C. Postischemic recovery of contractile function is impaired in SOD2(+/−) but not SOD1(+/−) mouse hearts. Circulation. 2002;105:981–6.
Abunasra HJ, Smolenski RT, Morrison K, et al. Efficacy of adenoviral gene transfer with manganese superoxide dismutase and endothelial nitric oxide synthase in reducing ischemia and reperfusion injury. Eur J Cardiothorac Surg. 2001;20:153–8.
Chen Z, Siu B, Ho YS, et al. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J Mol Cell Cardiol. 1998;30:2281–9.
Argaud L, Gateau-Roesch O, Chalabreysse L, et al. Preconditioning delays Ca2+-induced mitochondrial permeability transition. Cardiovasc Res. 2004;61:115–22.
Khaliulin I, Schwalb H, Wang P, et al. Preconditioning improves postischemic mitochondrial function and diminishes oxidation of mitochondrial proteins. Free Radic Biol Med. 2004;37:1–9.
Opie LH. Acute metabolic response in myocardial infarction. Br Heart J. 1971;33:129–37.
King LM, Opie LH. Glucose and glycogen utilisation in myocardial ischemia—changes in metabolism and consequences for the myocyte. Mol Cell Biochem. 1998;180:3–26.
Neubauer S. The failing heart—an engine out of fuel. N Engl J Med. 2007;356:1140–51.
Fields AV, Patterson B, Karnik AA, Shannon RP. Glucagon-like peptide-1 and myocardial protection: more than glycemic control. Clin Cardiol. 2009;32:236–43.
Chen CL, Chen J, Rawale S, et al. Protein tyrosine nitration of the flavin subunit is associated with oxidative modification of mitochondrial complex II in the post-ischemic myocardium. J Biol Chem. 2008;283:27991–8003.
Chen J, Henderson GI, Freeman GL. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J Mol Cell Cardiol. 2001;33:1919–27.
Paradies G, Petrosillo G, Pistolese M, Di Venosa N, Serena D, Ruggiero FM. Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radic Biol Med. 1999;27:42–50.
Forsmark-Andree P, Lee CP, Dallner G, Ernster L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic Biol Med. 1997;22:391–400.
Ide T, Tsutsui H, Hayashidani S, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–35.
Timmers L, Henriques JP, de Kleijn DP, et al. Exenatide reduces infarct size and improves cardiac function in a porcine model of ischemia and reperfusion injury. J Am Coll Cardiol. 2009;53:501–10.
Liu J, Yin F, Zheng X, Jing J, Hu Y. Geniposide, a novel agonist for GLP-1 receptor, prevents PC12 cells from oxidative damage via MAP kinase pathway. Neurochem Int. 2007;51:361–9.
Barker DJ, Osmond C, Law CM. The intrauterine and early postnatal origins of cardiovascular disease and chronic bronchitis. J Epidemiol Community Health. 1989;43:237–40.
Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80.
Osmond C, Barker DJ, Winter PD, Fall CH, Simmonds SJ. Early growth and death from cardiovascular disease in women. BMJ. 1993;307:1519–24.
Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348:1478–80.
Rich-Edwards JW, Colditz GA, Stampfer MJ, et al. Birthweight and the risk for type 2 diabetes mellitus in adult women. Ann Intern Med. 1999;130:278–84.
Curhan GC, Chertow GM, Willett WC, et al. Birth weight and adult hypertension and obesity in women. Circulation. 1996;94:1310–5.
Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–6.
Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–53.
Burdge GC, Hanson MA, Slater-Jefferies JL, Lillycrop KA. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br J Nutr. 2007;97:1036–46.
Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. 2007;61:5R–10.
Acknowledgements
This work was supported by the National Institutes of Health grant #DK55704 and AG20898 (RAS), and T32 HL 007843-12 (SBB). We thank Hongshun Niu for his expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brown, S.B., Libonati, J.R., Selak, M.A. et al. Neonatal Exendin-4 Leads to Protection from Reperfusion Injury and Reduced Rates of Oxidative Phosphorylation in the Adult Rat Heart. Cardiovasc Drugs Ther 24, 197–205 (2010). https://doi.org/10.1007/s10557-010-6242-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-010-6242-z