Abstract
Purpose
We tested whether upregulation of hypoxia inducible factor-1 (HIF-1) would restore the blunted effects of natriuretic peptides and nitric oxide caused by chronic nitrate exposure and stunning in cardiac myocytes.
Methods
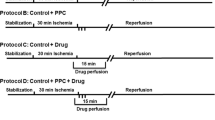
HIF-1α was increased with deferoxamine (150 mg/kg for 2 days). Nitrate tolerance was induced by a chronic nitroglycerin patch (0.3 mg/h for 5 days). We used freshly isolated rabbit ventricular myocytes. Half the myocytes were subjected to simulated ischemia [15 min 95% N2-5% CO2] and reperfusion [reoxygenation] to produce stunning. Cell function was measured utilizing a video-edge detector. Shortening was examined at baseline and after brain natriuretic peptide (BNP, 10−8, 10−7 M) or S-nitroso-N-acetyl-penicillamine (SNAP, 10−6, 10−5 M) followed by KT5823 (cyclic GMP protein kinase inhibitor, 10−6 M). We also measured cyclic GMP protein kinase protein levels and kinase activity.
Results
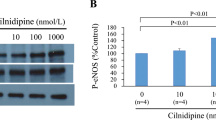
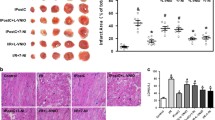
In control, BNP (−29%) reduced percent shortening, while KT5823 partially restored function. Deferoxamine treated control myocytes responded similarly. In patched nonstunned myocytes, BNP (−12%) reduced shortening less and KT5823 did not increase function. However, deferoxamine restored the blunted effects of BNP (−21%) and KT5823. In stunned myocytes, BNP (−11%) reduced shortening less and KT5823 did not affect function. Deferoxamine increased the effects of BNP (−27%) and KT5823 in stunning. Patch combined with stunning also similarly blunted the effects of BNP (−12%) and KT5823. Deferoxamine improved the effects of BNP (−22%) and KT5823. Similar results were observed after SNAP. Stunning reduced cyclic GMP protein kinase activity and deferoxamine restored activity. Deferoxamine had no effect on kinase activity in nitrate tolerance.
Conclusion
We found that upregulation of HIF-1 could protect isolated cardiac myocytes against nitrate tolerance through a cyclic GMP protein kinase-independent mechanism and through a kinase-dependent mechanism in stunning.








Similar content being viewed by others
References
Murad F. Cellular signaling with nitric oxide and cyclic GMP. Braz J Med Biol Res. 1999;32:1317–27.
Thadani U. Nitrate tolerance, rebound, and their clinical relevance in stable angina pectoris, unstable angina, and heart failure. Cardiovasc Drugs Ther. 1997;10:735–42.
Shah AM, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Ther. 2000;86:49–86.
Tan T, Zhang Q, Anyadike C, Scholz PM, Weiss HR. Chronic nitrates blunt the effects of not only nitric oxide but also natriuretic peptides in cardiac myocytes. Pharmacol Res. 2007;56:49–55.
Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97:618–28.
Heyndrickx GR. Myocardial stunning: an experimental act with a large clinical audience. Arch Mal Coeur Vaiss. 2003;96:665–70.
Anyadike C, Scholz PM, Zhang Q, Katz E, Weiss HR. Brain natriuretic peptide reverses the effects of myocardial stunning in rabbit myocardium. Pharmacology. 2007;80:40–8.
Zhang Q, Lazar M, Yan L, et al. Cyclic GMP reduces myocardial stunning through non-cyclic GMP protein kinase mechanisms. J Cardiovasc Pharmacol. 2004;44:235–43.
Dery MA, Michaud MD, Richard DE. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int J Biochem Cell Biol. 2005;37:535–40.
Poellinger L, Johnson RS. HIF-1 and hypoxic response: the plot thickens. Curr Opin Genet Dev. 2004;14:81–5.
Semenza GL. O2-regulated gene expression: transcriptional control of cardiorespiratory physiology by HIF-1. J Appl Physiol. 2004;96:1173–7. discussion 0–2.
Luciano JA, Tan T, Zhang Q, Huang E, Scholz P, Weiss HR. Hypoxia inducible factor-1 improves the actions of nitric oxide and natriuretic peptides after simulated ischemia-reperfusion. Cell Physiol Biochem. 2008;21:421–8.
Su J, Zhang Q, Moalem J, Tse J, Scholz PM, Weiss HR. Functional effects of C-type natriuretic peptide and nitric oxide are attenuated in hypertrophic myocytes from pressure-overloaded mouse hearts. Am J Physiol Heart Circ Physiol. 2005;288:H1367–73.
Zhu H, Cai C, Chen J. Suppression of P-glycoprotein gene expression in Hs578T/Dox by the overexpression of caveolin-1. FEBS letters. 2004;576:369–74.
Ziolo MT, Kohr MJ, Wang H. Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol. 2008;45:625–32.
Yan L, Zhang Q, Scholz PM, Weiss HR. Cyclic GMP protein kinase activity is reduced in thyroxine-induced hypertrophic cardiac myocytes. Clin Exp Pharmacol Physiol. 2003;30:943–50.
Francis SH, Blount MA, Zoraghi R, Corbin JD. Molecular properties of mammalian proteins that interact with cGMP: protein kinases, cation channels, phosphodiesterases, and multi-drug anion transporters. Front Biosci. 2005;10:2097–117.
Kojda G, Kottenberg K. Regulation of basal myocardial function by NO. Cardiovasc Res. 1999;41:514–23.
Klemenska E, Beresewicz A. Bioactivation of organic nitrates and the mechanism of nitrate tolerance. Cardiol J. 2009;16:11–9.
Gori T, Parker JD. Nitrate tolerance: a unifying hypothesis. Circulation. 2002;106:2510–3.
Mulsch A, Oelze M, Kloss S, et al. Effects of in vivo nitroglycerin treatment on activity and expression of the guanylyl cyclase and cGMP-dependent protein kinase and their downstream target vasodilator-stimulated phosphoprotein in aorta. Circulation. 2001;103:2188–94.
Sage PR, de la Lande IS, Stafford I, et al. Nitroglycerin tolerance in human vessels: evidence for impaired nitroglycerin bioconversion. Circulation. 2000;102:2810–5.
Warnholtz A, Tsilimingas N, Wendt M, Munzel T. Mechanisms underlying nitrate-induced endothelial dysfunction: insight from experimental and clinical studies. Heart Fail Rev. 2002;7:335–45.
Barnes E, Khan MA. Myocardial stunning in man. Heart Fail Rev. 2003;8:155–60.
Gowda RM, Khan IA, Vasavada BC, Sacchi TJ. Reversible myocardial dysfunction: basics and evaluation. Int J Cardiol. 2004;97:349–53.
Weiss HR, Gandhi A, Scholz PM. Negative effect of nitric oxide on shortening-frequency relationship in cardiac myocytes is diminished after simulated ischemia-reperfusion. Basic Res Cardiol. 2003;98:311–8.
Hannan RL, John MC, Kouretas PC, Hack BD, Matherne GP, Laubach VE. Deletion of endothelial nitric oxide synthase exacerbates myocardial stunning in an isolated mouse heart model. J Surg Res. 2000;93:127–32.
Brune B, Zhou J. Nitric oxide and superoxide: interference with hypoxic signaling. Cardiovasc Res. 2007;75:275–82.
Shrivastava K, Shukla D, Bansal A, Sairam M, Banerjee PK, Ilavazhagan G. Neuroprotective effect of cobalt chloride on hypobaric hypoxia-induced oxidative stress. Neurochem Int. 2008;52:368–75.
Kirito K, Hu Y, Komatsu N. HIF-1 prevents the overproduction of mitochondrial ROS after cytokine stimulation through induction of PDK-1. Cell Cycle. 2009;8:2844–9.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, T., Weiss, H.R. Hypoxia Inducible Factor-1 Protects Against Nitrate Tolerance and Stunning in Rabbit Cardiac Myocytes. Cardiovasc Drugs Ther 24, 95–106 (2010). https://doi.org/10.1007/s10557-010-6229-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-010-6229-9