Abstract
Purpose
AMPK plays a crucial role in the regulation of the energy metabolism of the heart. During ischaemia, AMPK activation is a known adaptative prosurvival mechanism that helps to maintain the energy levels of the myocardium. However, it still remains unclear if activation of AMPK during reperfusion is beneficial for the heart. Two known AMPK activators (metformin and AICAR) were used to verify the hypothesis that a transitory activation of AMPK at reperfusion may exert cardioprotection, as reflected in a reduction in myocardial infarct size.
Methods
Perfused rat hearts were subjected to 35 min ischaemia and 120 min reperfusion. Metformin (50 μM) or AICAR (0.5 mM) were added for 15 min at the onset of reperfusion alone or with Compound C (CC, 10 μM), an AMPK inhibitor. Infarct size and α-AMPK phosphorylation were measured.
Results
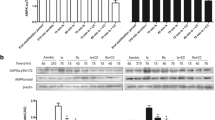
Metformin significantly reduced infarct size from 47.8 ± 1.7% in control to 31.4 ± 2.9%, an effect abolished by CC when the drugs were given concomitantly. Similarly, AICAR also induced a significant reduction in infarct size to 32.3 ± 4.8%, an effect also abrogated by CC. However, metformin’s protection was not abolished if CC was administered later in reperfusion. In addition, α-AMPK phosphorylation was significantly increased in the metformin treated group during the initial 30 min of reperfusion.
Conclusions
Our data demonstrated that, in our ex vivo model of myocardial ischaemia-reperfusion injury, AMPK activation in early reperfusion is associated with a reduction in infarct size.





Similar content being viewed by others
References
Hardie DG. Role of AMP-activated protein kinase in the metabolic syndrome and in heart disease. FEBS Lett. 2008;582:81–9.
Wong KA, Lodish HF. A revised model for AMP-activated protein kinase structure: the alpha-subunit binds to both the beta- and gamma-subunits although there is no direct binding between the beta- and gamma-subunits. J Biol Chem. 2006;281:36434–42.
Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89:1025–78.
Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, et al. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest. 2003;111:91–8.
Lopaschuk GD. AMP-activated protein kinase control of energy metabolism in the ischemic heart. Int J Obes (Lond). 2008;32:S29–35.
Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65.
Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574–9.
Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74.
Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348:607–14.
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 854–65.
Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, et al. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–84.
Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, et al. Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 2008;57:696–705.
Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Monteiro P, Goncalves L, et al. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol. 2009;53:373–8.
Tsuchida A, Yang XM, Burckhartt B, Mullane KM, Cohen MV, Downey JM. Acadesine extends the window of protection afforded by ischaemic preconditioning. Cardiovasc Res. 1994;28:379–83.
Wynne AM, Mocanu MM, Yellon DM. Pioglitazone mimics preconditioning in the isolated perfused rat heart: a role for the prosurvival kinases PI3K and P42/44MAPK. J Cardiovasc Pharmacol. 2005;46:817–22.
Legtenberg RJ, Houston RJ, Oeseburg B, Smits P. Metformin improves cardiac functional recovery after ischemia in rats. Horm Metab Res. 2002;34:182–5.
Leon H, Atkinson LL, Sawicka J, Strynadka K, Lopaschuk GD, Schulz R. Pyruvate prevents cardiac dysfunction and AMP-activated protein kinase activation by hydrogen peroxide in isolated rat hearts. Can J Physiol Pharmacol. 2004;82:409–16.
Mocanu MM, Bell RM, Yellon DM. PI3 kinase and not p42/p44 appears to be implicated in the protection conferred by ischemic preconditioning. J Mol Cell Cardiol. 2002;34:661–8.
Sasaki H, Asanuma H, Fujita M, Takahama H, Wakeno M, Ito S, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation 2009;119:2568–77.
Russell 3rd RR, Li J, Coven DL, Pypaert M, Zechner C, Palmeri M, et al. AMP-activated protein kinase mediates ischemic glucose uptake and prevents postischemic cardiac dysfunction, apoptosis, and injury. J Clin Invest. 2004;114:495–503.
Folmes CD, Wagg CS, Shen M, Clanachan AS, Tian R, Lopaschuk GD. Suppression of 5′-AMP-activated protein kinase activity does not impair recovery of contractile function during reperfusion of ischemic hearts. Am J Physiol Heart Circ Physiol. 2009;297:H313–21.
Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, et al. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK-and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–103.
Kawabata H, Ishikawa K. Cardioprotection by metformin is abolished by a nitric oxide synthase inhibitor in ischemic rabbit hearts. Hypertens Res. 2003;26:107–10.
Shin EJ, Schram K, Zheng XL, Sweeney G. Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol. 2009;221:490–7.
Huisamen B, Genade S, Lochner A. Signalling pathways activated by glucagon-like peptide-1 (7–36) amide in the rat heart and their role in protection against ischaemia. Cardiovasc J Afr. 2008;19:77–83.
Zhang L, He H, Balschi JA. Metformin and phenformin activate AMP-activated protein kinase in the heart by increasing cytosolic AMP concentration. Am J Physiol Heart Circ Physiol. 2007;293:H457–66.
Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WG 4th, Schlattner U, et al. Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem. 2004;279:43940–51.
Zhang L, Frederich M, He H, Balschi JA. Relationship between 5-aminoimidazole-4-carboxamide-ribotide and AMP-activated protein kinase activity in the perfused mouse heart. Am J Physiol Heart Circ Physiol. 2006;290:H1235–43.
Cohen MV, Yang XM, Downey JM. The pH hypothesis of postconditioning: staccato reperfusion reintroduces oxygen and perpetuates myocardial acidosis. Circulation 2007;115:1895–903.
Dyck JR, Lopaschuk GD. AMPK alterations in cardiac physiology and pathology: enemy or ally? J Physiol. 2006;574:95–112.
Moopanar TR, Xiao XH, Jiang L, Chen ZP, Kemp BE, Allen DG. AICAR inhibits the Na+/H+ exchanger in rat hearts-possible contribution to cardioprotection. Pflugers Arch. 2006;453:147–56.
King TD, Song L, Jope RS. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem Pharmacol. 2006;28:1637–47.
Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese Jr RV, et al. Activation of the ERK pathway and atypical protein kinase C isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-D-riboside (AICAR)-stimulated glucose transport. J Biol Chem. 2002;28:23554–62.
Saeedi R, Parsons HL, Wambolt RB, Paulson K, Sharma V, Dyck JR, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am J Physiol Heart Circ Physiol. 2008;294:H2497–506.
Lopaschuk GD, Belke DD, Gamble J, Itoi T, Schonekess BO. Regulation of fatty acid oxidation in the mammalian heart in health and disease. Biochim Biophys Acta. 1994;1213:263–76.
Liu Q, Docherty JC, Rendell JC, Clanachan AS, Lopaschuk GD. High levels of fatty acids delay the recovery of intracellular pH and cardiac efficiency in post-ischemic hearts by inhibiting glucose oxidation. J Am Coll Cardiol. 2002;39:718–25.
Clark H, Carling D, Saggerson D. Covalent activation of heart AMP-activated protein kinase in response to physiological concentrations of long-chain fatty acids. Eur J Biochem. 2004;271:2215–24.
Solskov L, Lofgren B, Kristiansen SB, Jessen N, Pold R, Nielsen TT, et al. Metformin induces cardioprotection against ischaemia/reperfusion injury in the rat heart 24 h after administration. Basic Clin Pharmacol Toxicol. 2008;103:82–7.
Acknowledgments
MA Paiva is a recipient of a fellowship by Fundação para a Ciência e Tecnologia (SFRH/BD/23671/2005). We thank British Heart Foundation for continuous support.
Conflict of interest
The authors state no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paiva, M.A., Gonçalves, L.M., Providência, L.A. et al. Transitory Activation of AMPK at Reperfusion Protects the Ischaemic-Reperfused Rat Myocardium Against Infarction. Cardiovasc Drugs Ther 24, 25–32 (2010). https://doi.org/10.1007/s10557-010-6222-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-010-6222-3