Summary
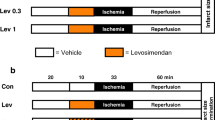
Introduction: Apoptosis occurring during ischaemia /reperfusion contributes independently to tissue damage, and involves activation of the stress-kinase, p38 MAPK during reperfusion. Ischaemic preconditioning (IPC) protects against ischaemia/reperfusion mediated necrosis and apoptosis. The role of p38 MAPK in the protective effect of preconditioning against apoptosis is unknown. Pharmacologic preconditioning with isoproterenol (β-PC) also protects against necrosis, but it is not known whether it protects against apoptosis. Aim: The aim of the study was to investigate whether the protective effect of IPC against apoptosis is related to activation of p38 MAPK and whether β-PC also protects against apoptosis. Materials and methods: Isolated perfused rat hearts were used to study the effect of ischaemia and reperfusion on apoptosis and infarct size. Ischaemic preconditioning was elicited by 3 × 5 min global ischaemia, and β-PC by 5 min isoproterenol 10−7 M. For infarct size hearts were subjected to regional ischaemia for 35 min followed by 120 min reperfusion. Infarct size was determined by the tetrazolium staining technique, and expressed as percentage of area at risk. For markers of apoptosis hearts were subjected to global ischaemia of 25 min plus 30 min reperfusion. Apoptosis was determined by Western blot using antibodies against caspase-3 and PARP. p38 MAPK activation was inhibited by SB203580 (1 μM) administration 10 min prior to commencing ischaemia, and bracketing the IPC and β-PC preconditioning protocols. p38 MAPK was activated by administration of anisomycin (5 μM) 10 min prior to index ischaemia in one protocol, and 10 min during reperfusion in non-preconditioned as well as IPC and β-PC hearts. Results were analysed using ANOVA and a Newman-Keuls post-hoc test. Results: In the apoptosis model using global ischaemia, IPC and β-PC both resulted in a significant decrease in p38 MAPK activation at the end of reperfusion when compared to non-preconditioned hearts. This was accompanied by a significant decrease in apoptosis as measured with both caspase-3 activation and PARP cleavage. Inhibiting p38 MAPK by administration of SB203580 10 min prior to ischaemia resulted in a significant reduction in both markers of apoptosis. Bracketing the triggering phase of either IPC or β-PC with SB203580 resulted in attenuated p38 MAPK activation during reperfusion and did not abolish the protective effect of IPC or β-PC against apoptosis. Activating p38 MAPK with anisomycin prior to ischaemia resulted in a reduction of markers of apoptosis, whereas activation of p38 MAPK with anisomycin during reperfusion did not exacerbate apoptosis in any groups, exept for an increase in PARP cleavage in IPC hearts. In the model of regional ischaemia, IPC and β-PC reduced infarct size significantly, and to the same extent as inhibition of p38 MAPK by administration of SB203580 10 min prior to ischaemia. Bracketing the triggering phase of either IPC or β-PC did not abolish the reduction in infarct size. Activating p38 MAPK during reperfusion was accompanied by an increase in infarct size only in IPC hearts, but not in β-PC hearts. Conclusion: These results indicate that (1) Both IPC and β-PC elicit protection against apoptosis and necrosis, (2) activation of p38 MAPK is not a trigger of preconditioning against apoptosis and necrosis and (3) activation of p38 MAPK during reperfusion increases necrosis only if ischaemia is used to precondition hearts, but not with pharmacologic preconditioning with isoproterenol.
Similar content being viewed by others
References
Gottlieb RRA, Engler RL. Apoptosis in myocardial ischemia-reperfusion. Ann NY Acad Sci 1999;874:412–426.
Freude B, Masters TN, Robicsek F, et al. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol 2000;32:197–208.
Krijnen PAJ, Nijmeijer R, Meijer CJLM, Hack CA, Niessen HWM. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol 2002;55:801–811.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: A delay of lethal injury in ischemic myocardium. Circulation 1986;74:1124–1136.
Miura T, Ishimoto R, Sakamoto J, et al. Suppression of reperfusion arrhythmia by ischemic preconditioning in the rat: Is it mediated by the adenosine receptor, prostaglandin, or bradykinin receptor? Basic Res Cardiol 1995;90:240–246.
Nakamura M, Wang NP, Zhao ZQ, et al. Preconditioning decreases Bax expression, PMN accumulation and apoptosis in reperfused rat hearts. Cardiovasc Res 2000;45:661–670.
Piot CA, Padmanabam D, Ursell PC, Sievers RE, Wolfe CL. Ischemic preconditioning decreases apoptosis in rat hearts in vivo. Circulation 1997;96:1598–1604.
Zhao ZQ, Vinten-Johansen J. Myocardial apoptosis and ischemic preconditioning. Cardiovasc Res 2002;55:438–455.
Ambrosio G, Zweier JL, Becker LC. Apoptosis is prevented by administration of superoxide dismutase in dogs with reperfused myocardial infarction. Basic Res Cardiol 1998;93:94–96.
Freude B, Masters TN, Kostin S, Robicsek F, Schaper J. Cardiomyocyte apoptosis in acute and chronic conditions. Basic Res Cardiol 1998;93:85–89.
Maulik N, Engelman RM, Rousou JA, Flack JE III, Deaton D, Das DK. Ischemic preconditioning reduces apoptosis by upregulating anti-death gene Bcl-2. Circulation 1999;100(19 Suppl):II369–II375.
Piot CA, Martini JF, Bui SK, Wolfe CL. Ischemic preconditioning attenuates ischemia/reperfusion-induced activation of caspases and subsequent cleavage of poly (ADP-ribose) polymerase in rat hearts in vivo. Cardiovasc Res 1999;44:536–542.
Argaud L, Prigent AF, Chalabreysse L, Loufouat J, Lagarde M, Ovize M. Ceramide in the antiapoptotic effect of ischemic preconditioning. Am J Physiol Heart Circ Physiol 2004;286:H246–H251.
Okamura T, Miura T, Iwamoto H, et al. Ischemic preconditioning attenuates apoptosis through protein kinase C in rat hearts. Am J Physiol 1999;277:H1997–H2001.
Kyriakis JM, Banerjee P, Nikolakaki E, et al. The stress-activated protein kinase subfamily of c-Jun kinases. Nature 1994;369:156–160.
Yin T, Sandhu G, Wolfgang CD, et al. Tissue-specific patterns of stress kinase activation in ischemic/reperfused heart and kidney. J Biol Chem 1997;272:19943–19950.
Ma XL, Kumar S, Gao F, et al. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 1999;99:1685–1691.
Han H, Wang H, Long H, Nattel S, Wang Z. Oxidative preconditioning and apoptosis in L-cells. Roles of protein kinase B and mitogen-activated protein kinases. J Biol Chem 2001;276:26357–26364.
Zhang S, Ren J, Zhang CE, Treskov I, Wang Y, Muslin AJ. Role of 14-3-3-mediated p38 mitogen-activated protein kinase inhibition in cardiac myocyte survival. Circ Res 2003;93:1026–1028.
Steenbergen C. The role of p38 mitogen-activated protein kinase in myocardial ischemia/reperfusion injury; relationship to ischemic preconditioning. Basic Res Cardiol 2002;97:276–285.
Marais E, Genade S, Huisamen B, Strijdom JG, Moolman JA. Activation of p38 MAPK induced by a multi-cycle ischaemic reconditioning protocol is associated with attenuated p38 MAPK activity during sustained ischaemia and reperfusion. J Moll Cell Cardiol 2001;33:769–778.
Schneiders S, Chen W, Hou J, Steenbergen C, Murphy E. Inhibition of p38 MAPK a/b reduces ischemic injury and does not block the protective effects of preconditioning. Am J Physiol 2001;280:H499–H580.
Lochner A, Marais E, Genade S, Huisamen B, Strijdom JG, Moolman JA. Ischemic and pharmacological preconditioning is associated with attenuation of p38 MAPK activation during sustained ischemia and reperfusion. In: Dhalla NS, Takeda N, Lukas A, eds. Myocardial Ischemia and Preconditioning. Boston: Kluwer Academic Publishers, 2002:249–273.
Marais E, Genade S, Strijdom JG, Moolman JA, Lochner A. p38 MAPK activation triggers pharmacologically-induced beta-adrenergic preconditioning, but not ischaemic preconditioning. J Moll Cell Cardiol 2001;33:2157–2177.
Yellon DM, Downey JM. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol Rev 2003;83:1113–1151.
McCully JD, Wakiyama H, Hsieh Y, Jones M, Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 2004;286:H1923–H1935.
Martin JL, Avkiram M, Quinlan RA, Cohen P, Marber MS. Anti-ischemic effects of SB203580 are mediated through the inhibition of p38 alpha mitogen-activated protein kinase: Evidence from ectopic expression of an inhibition-resistant kinase. Circ Res 2001;89:750–752.
Otsu K, Yamashita N, Nishida K, et al. Disruption of a single copy of the p38alpha MAP kinase gene leads to cardioprotection against ischemia/reperfusion. Biochem Biophys Res Commun 2003;302:56–60.
Clerk A, Sugden PH. The p38-MAPK inhibitor, SB203580, inhibits cardiac stress-activated protein kinases/cJun N-terminal kinases (SAPKs/JNKs). FEBS Lett 1998;426:93–96.
Lochner A, Genade S, Moolman JA. Ischemic preconditioning: Infarct size is a more reliable endpoint than functional recovery. Basic Res Cardiol 2003;98:337–346.
Martin JL, Mestril R, Hilal-Dandan R, Brunton LL, Dillmann WH. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation 1997;96:4343–4348.
Zhu WZ, Wang SQ, Chakir K, et al. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J Clin Invest 2003;111:617–625.
Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, Xiao RP. Dual modulation of cell survival and cell death by beta(2)-adrenergic signaling in adult mouse cardiac myocytes. Proc Natl Acad Sci USA 2001;98:1607–1612.
Tong H, Bernstein D, Murphy E, Steenbergen C. The role of β-adrenergic receptor signaling in cardioprotection. FASEB J 2005;19(8):983–985.
Frances C, Nazeyrollas P, Prevost P, et al. Role of beta-1 and beta-2 adrenoreceptor subtypes in preconditioning against myocardial dysfunction after ischaemia and reperfusion. J Cardiovasc Pharmacol 2003;41(3):396–405.
Lochner A, Genade S, Tromp E, Podzuweit T, Moolman JA. Ischemic preconditioning and the beta-adrenergic signal transduction pathway. Circulation 1999;100:958–966.
Barancik M, Htun P, Schaper W. Okadaic acid and anisomycin are protective and stimulate the SAPK/JNK pathway. J Cardiovasc Pharmacol 1999;34:182–190.
Mackay K, Mochly-Rosen D. An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J Biol Chem 1999;274:6272–6279.
Lochner A, Genade S, Hattingh S, Marais E, Huisamen B, Moolman JA. Comparison between ischaemic and anisomycin-induced preconditioning: Role of p38 MAPK. Cardiovasc Drugs Ther 2003;17(3):217–230.
Martindale JJ, Wall JA, Martinez-Longoria DM, et al. Overexpression of mitogen-activated protein kinase 6 in the heart improves functional recovery from ischemia in vitro and protects against myocardial infarction in vivo. JBC 2005;280(1):669–676.
Tanno M, Bassi R, Gorog DA, et al. Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation. Circ Res 2003;93:254–261.
Zhu L, Fukuda S, Cordis G, Das DK, Maulik N. Anti-apoptotic protein survivin plays a significant role in tubular morphogenesis of human coronary arteriolar endothelial cells by hypoxic preconditioning. FEBS Lett 2001;23,508(3):369–374.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moolman, J.A., Hartley, S., Van Wyk, J. et al. Inhibition of Myocardial Apoptosis by Ischaemic and Beta-Adrenergic Preconditioning is Dependent on p38 MAPK. Cardiovasc Drugs Ther 20, 13–25 (2006). https://doi.org/10.1007/s10557-006-6257-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-006-6257-7