Abstract
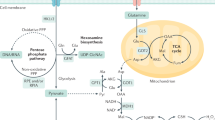
Pancreatic cancer is a paradigm for adaptation to extreme stress. That is because genetic drivers are selected during tissue injury with epigenetic imprints encoding wound healing responses. Ironically, epigenetic memories of trauma that facilitate neoplasia can also recreate past stresses to restrain malignant progression through symbiotic tumor:stroma crosstalk. This is best exemplified by positive feedback between neoplastic chromatin outputs and fibroinflammatory stromal cues that encase malignant glands within a nutrient-deprived desmoplastic stroma. Because epigenetic imprints are chemically encoded by nutrient-derived metabolites bonded to chromatin, primary tumor metabolism adapts to preserve malignant epigenetic fidelity during starvation. Despite these adaptations, stromal stresses inevitably awaken primordial drives to seek more hospitable climates. The invasive migrations that ensue facilitate entry into the metastatic cascade. Metastatic routes present nutrient-replete reservoirs that accelerate malignant progression through adaptive metaboloepigenetics. This is best exemplified by positive feedback between biosynthetic enzymes and nutrient transporters that saturate malignant chromatin with pro-metastatic metabolite byproducts. Here we present a contemporary view of pancreatic cancer epigenetics: selection of neoplastic chromatin under fibroinflammatory pressures, preservation of malignant chromatin during starvation stresses, and saturation of metastatic chromatin by nutritional excesses that fuel lethal metastasis.





Similar content being viewed by others
References
Schvartzman, J. M., Thompson, C. B., & Finley, L. W. S. (2018). Metabolic regulation of chromatin modifications and gene expression. Journal of Cell Biology, 217(7), 2247–2259. https://doi.org/10.1083/jcb.201803061
Cavalli, G., & Heard, E. (2019). Advances in epigenetics link genetics to the environment and disease. Nature, 571(7766), 489–499. https://doi.org/10.1038/s41586-019-1411-0
Kinnaird, A., Zhao, S., Wellen, K. E., & Michelakis, E. D. (2016). Metabolic control of epigenetics in cancer. Nature Reviews Cancer, 16(11), 694–707. https://doi.org/10.1038/nrc.2016.82
Strahl, B. D., & Allis, C. D. (2000). The language of covalent histone modifications. Nature, 403(6765), 41–45. https://doi.org/10.1038/47412
Boon, R., Silveira, G. G., & Mostoslavsky, R. (2020). Nuclear metabolism and the regulation of the epigenome. Nature Metabolism, 2(11), 1190–1203. https://doi.org/10.1038/s42255-020-00285-4
Reid, M. A., Dai, Z., & Locasale, J. W. (2017). The impact of cellular metabolism on chromatin dynamics and epigenetics. Nature Cell Biology, 19(11), 1298–1306. https://doi.org/10.1038/ncb3629
Ushijima, T., Clark, S. J., & Tan, P. (2021). Mapping genomic and epigenomic evolution in cancer ecosystems. Science, 373(6562), 1474–1479. https://doi.org/10.1126/science.abh1645
Locasale, J. W. (2018). New concepts in feedback regulation of glucose metabolism. Curr Opin Syst Biol, 8, 32–38. https://doi.org/10.1016/j.coisb.2017.11.005
Thompson, C. B., & Bielska, A. A. (2019). Growth factors stimulate anabolic metabolism by directing nutrient uptake. Journal of Biological Chemistry, 294(47), 17883–17888. https://doi.org/10.1074/jbc.AW119.008146
Halbrook, C. J., Lyssiotis, C. A., Pasca di Magliano, M., & Maitra, A. (2023). Pancreatic cancer: Advances and challenges. Cell, 186(8), 1729–1754. https://doi.org/10.1016/j.cell.2023.02.014
Encarnación-Rosado, J., & Kimmelman, A. C. (2021). Harnessing metabolic dependencies in pancreatic cancers. Nature Reviews. Gastroenterology & Hepatology, 18(7), 482–492. https://doi.org/10.1038/s41575-021-00431-7
Halbrook, C. J., & Lyssiotis, C. A. (2017). Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell, 31(1), 5–19. https://doi.org/10.1016/j.ccell.2016.12.006
Rahib, L., Wehner, M. R., Matrisian, L. M., & Nead, K. T. (2021). Estimated projection of US cancer incidence and death to 2040. JAMA Netw Open, 4(4), e214708. https://doi.org/10.1001/jamanetworkopen.2021.4708
Hayashi, A., Hong, J., & Iacobuzio-Donahue, C. A. (2021). The pancreatic cancer genome revisited. Nature Reviews. Gastroenterology & Hepatology, 18(7), 469–481. https://doi.org/10.1038/s41575-021-00463-z
Sandgren, E. P., Luetteke, N. C., Palmiter, R. D., Brinster, R. L., & Lee, D. C. (1990). Overexpression of TGF alpha in transgenic mice: Induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell, 61(6), 1121–1135. https://doi.org/10.1016/0092-8674(90)90075-p
Halbrook, C. J., Wen, H. J., Ruggeri, J. M., Takeuchi, K. K., Zhang, Y., di Magliano, M. P., et al. (2017). Mitogen-activated protein kinase kinase activity maintains acinar-to-ductal metaplasia and is required for organ regeneration in pancreatitis. Cellular and Molecular Gastroenterology and Hepatology, 3(1), 99–118. https://doi.org/10.1016/j.jcmgh.2016.09.009
McDonald, O. G. (2022). The biology of pancreatic cancer morphology. Pathology, 54(2), 236–247. https://doi.org/10.1016/j.pathol.2021.09.012
Del Poggetto, E., Ho, I. L., Balestrieri, C., Yen, E. Y., Zhang, S., Citron, F., et al. (2021). Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science, 373(6561), eabj0486. https://doi.org/10.1126/science.abj0486
Alonso-Curbelo, D., Ho, Y. J., Burdziak, C., Maag, J. L. V., Morris, J. P. T., Chandwani, R., et al. (2021). A gene-environment-induced epigenetic program initiates tumorigenesis. Nature, 590(7847), 642–648. https://doi.org/10.1038/s41586-020-03147-x
Cobo, I., Martinelli, P., Flández, M., Bakiri, L., Zhang, M., Carrillo-de-Santa-Pau, E., et al. (2018). Transcriptional regulation by NR5A2 links differentiation and inflammation in the pancreas. Nature, 554(7693), 533–537. https://doi.org/10.1038/nature25751
Mallen-St Clair, J., Soydaner-Azeloglu, R., Lee, K. E., Taylor, L., Livanos, A., Pylayeva-Gupta, Y., et al. (2012). EZH2 couples pancreatic regeneration to neoplastic progression. Genes & Development, 26(5), 439–444. https://doi.org/10.1101/gad.181800.111
Hill, W., Zaragkoulias, A., Salvador-Barbero, B., Parfitt, G. J., Alatsatianos, M., Padilha, A., et al. (2021). EPHA2-dependent outcompetition of KRASG12D mutant cells by wild-type neighbors in the adult pancreas. Current Biology, 31(12), 2550-2560.e2555. https://doi.org/10.1016/j.cub.2021.03.094
Mathison, A. J., Kerketta, R., de Assuncao, T. M., Leverence, E., Zeighami, A., Urrutia, G., et al. (2021). Kras(G12D) induces changes in chromatin territories that differentially impact early nuclear reprogramming in pancreatic cells. Genome Biology, 22(1), 289. https://doi.org/10.1186/s13059-021-02498-6
Tape, C. J., Ling, S., Dimitriadi, M., McMahon, K. M., Worboys, J. D., Leong, H. S., et al. (2016). Oncogenic KRAS regulates tumor cell signaling via stromal reciprocation. Cell, 165(4), 910–920. https://doi.org/10.1016/j.cell.2016.03.029
Das, S., Shapiro, B., Vucic, E. A., Vogt, S., & Bar-Sagi, D. (2020). Tumor cell-derived IL1β promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Research, 80(5), 1088–1101. https://doi.org/10.1158/0008-5472.Can-19-2080
Pylayeva-Gupta, Y., Lee, K. E., Hajdu, C. H., Miller, G., & Bar-Sagi, D. (2012). Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell, 21(6), 836–847. https://doi.org/10.1016/j.ccr.2012.04.024
Hruban, R. H., Goggins, M., Parsons, J., & Kern, S. E. (2000). Progression model for pancreatic cancer. Clinical Cancer Research, 6(8), 2969–2972.
Notta, F., Chan-Seng-Yue, M., Lemire, M., Li, Y., Wilson, G. W., Connor, A. A., et al. (2016). A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature, 538(7625), 378–382. https://doi.org/10.1038/nature19823
Tajan, M., Hock, A. K., Blagih, J., Robertson, N. A., Labuschagne, C. F., Kruiswijk, F., et al. (2018). A role for p53 in the adaptation to glutamine starvation through the expression of SLC1A3. Cell Metabolism, 28(5), 721-736.e726. https://doi.org/10.1016/j.cmet.2018.07.005
Morris, J. P. T., Yashinskie, J. J., Koche, R., Chandwani, R., Tian, S., Chen, C. C., et al. (2019). α-Ketoglutarate links p53 to cell fate during tumour suppression. Nature, 573(7775), 595–599. https://doi.org/10.1038/s41586-019-1577-5
Koivunen, P., Hirsilä, M., Remes, A. M., Hassinen, I. E., Kivirikko, K. I., & Myllyharju, J. (2007). Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: Possible links between cell metabolism and stabilization of HIF. Journal of Biological Chemistry, 282(7), 4524–4532. https://doi.org/10.1074/jbc.M610415200
Zhu, J., Sammons, M. A., Donahue, G., Dou, Z., Vedadi, M., Getlik, M., et al. (2015). Gain-of-function p53 mutants co-opt chromatin pathways to drive cancer growth. Nature, 525(7568), 206–211. https://doi.org/10.1038/nature15251
Bachem, M. G., Schünemann, M., Ramadani, M., Siech, M., Beger, H., Buck, A., et al. (2005). Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology, 128(4), 907–921. https://doi.org/10.1053/j.gastro.2004.12.036
Öhlund, D., Handly-Santana, A., Biffi, G., Elyada, E., Almeida, A. S., Ponz-Sarvise, M., et al. (2017). Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. Journal of Experimental Medicine, 214(3), 579–596. https://doi.org/10.1084/jem.20162024
Tian, C., Clauser, K. R., Öhlund, D., Rickelt, S., Huang, Y., Gupta, M., et al. (2019). Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proc Natl Acad Sci U S A, 116(39), 19609–19618. https://doi.org/10.1073/pnas.1908626116
Provenzano, P. P., Cuevas, C., Chang, A. E., Goel, V. K., Von Hoff, D. D., & Hingorani, S. R. (2012). Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell, 21(3), 418–429. https://doi.org/10.1016/j.ccr.2012.01.007
Lee, S. W., Zhang, Y., Jung, M., Cruz, N., Alas, B., & Commisso, C. (2019). EGFR-Pak signaling selectively regulates glutamine deprivation-induced macropinocytosis. Developmental Cell, 50(3), 381-392.e385. https://doi.org/10.1016/j.devcel.2019.05.043
Kamphorst, J. J., Nofal, M., Commisso, C., Hackett, S. R., Lu, W., Grabocka, E., et al. (2015). Human pancreatic cancer tumors are nutrient poor and tumor cells actively scavenge extracellular protein. Cancer Research, 75(3), 544–553. https://doi.org/10.1158/0008-5472.Can-14-2211
Sullivan, M. R., Danai, L. V., Lewis, C. A., Chan, S. H., Gui, D. Y., Kunchok, T., et al. (2019). Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife, 8, e44235. https://doi.org/10.7554/eLife.44235
Hollinshead, K. E. R., Parker, S. J., Eapen, V. V., Encarnacion-Rosado, J., Sohn, A., Oncu, T., et al. (2020). Respiratory supercomplexes promote mitochondrial efficiency and growth in severely hypoxic pancreatic cancer. Cell Rep, 33(1), 108231. https://doi.org/10.1016/j.celrep.2020.108231
Kamphorst, J. J., Cross, J. R., Fan, J., de Stanchina, E., Mathew, R., White, E. P., et al. (2013). Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc Natl Acad Sci U S A, 110(22), 8882–8887. https://doi.org/10.1073/pnas.1307237110
Kerk, S. A., Papagiannakopoulos, T., Shah, Y. M., & Lyssiotis, C. A. (2021). Metabolic networks in mutant KRAS-driven tumours: Tissue specificities and the microenvironment. Nature Reviews Cancer, 21(8), 510–525. https://doi.org/10.1038/s41568-021-00375-9
Lo, E. K. W., Mears, B. M., Maurer, H. C., Idrizi, A., Hansen, K. D., Thompson, E. D., et al. (2023). Comprehensive DNA methylation analysis indicates that pancreatic intraepithelial neoplasia lesions are acinar-derived and epigenetically primed for carcinogenesis. Cancer Research. https://doi.org/10.1158/0008-5472.Can-22-4052
Lomberk, G., Dusetti, N., Iovanna, J., & Urrutia, R. (2019). Emerging epigenomic landscapes of pancreatic cancer in the era of precision medicine. Nature Communications, 10(1), 3875. https://doi.org/10.1038/s41467-019-11812-7
Collisson, E. A., Bailey, P., Chang, D. K., & Biankin, A. V. (2019). Molecular subtypes of pancreatic cancer. Nature Reviews. Gastroenterology & Hepatology, 16(4), 207–220. https://doi.org/10.1038/s41575-019-0109-y
Hayashi, A., Fan, J., Chen, R., Ho, Y.-J., Makohon-Moore, A. P., Lecomte, N., et al. (2020). A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nature Cancer, 1(1), 59–74. https://doi.org/10.1038/s43018-019-0010-1
Noë, M., Hong, S. M., Wood, L. D., Thompson, E. D., Roberts, N. J., Goggins, M. G., et al. (2021). Pancreatic cancer pathology viewed in the light of evolution. Cancer and Metastasis Reviews. https://doi.org/10.1007/s10555-020-09953-z
Tu, M., Klein, L., Espinet, E., Georgomanolis, T., Wegwitz, F., Li, X., et al. (2021). TNF-α-producing macrophages determine subtype identity and prognosis via AP1 enhancer reprogramming in pancreatic cancer. Nat Cancer, 2(11), 1185–1203. https://doi.org/10.1038/s43018-021-00258-w
Somerville, T. D., Biffi, G., Daßler-Plenker, J., Hur, S. K., He, X. Y., Vance, K. E., et al. (2020). Squamous trans-differentiation of pancreatic cancer cells promotes stromal inflammation. Elife, 9, e53381. https://doi.org/10.7554/eLife.53381
Somerville, T. D. D., Xu, Y., Miyabayashi, K., Tiriac, H., Cleary, C. R., Maia-Silva, D., et al. (2018). TP63-mediated enhancer reprogramming drives the squamous subtype of pancreatic ductal adenocarcinoma. Cell Reports, 25(7), 1741-1755.e1747. https://doi.org/10.1016/j.celrep.2018.10.051
Lomberk, G., Blum, Y., Nicolle, R., Nair, A., Gaonkar, K. S., Marisa, L., et al. (2018). Distinct epigenetic landscapes underlie the pathobiology of pancreatic cancer subtypes. Nature Communications, 9(1), 1978. https://doi.org/10.1038/s41467-018-04383-6
Lee, A. Y. L., Dubois, C. L., Sarai, K., Zarei, S., Schaeffer, D. F., Sander, M., et al. (2019). Cell of origin affects tumour development and phenotype in pancreatic ductal adenocarcinoma. Gut, 68(3), 487–498. https://doi.org/10.1136/gutjnl-2017-314426
Brunton, H., Caligiuri, G., Cunningham, R., Upstill-Goddard, R., Bailey, U. M., Garner, I. M., et al. (2020). HNF4A and GATA6 loss reveals therapeutically actionable subtypes in pancreatic cancer. Cell Rep, 31(6), 107625. https://doi.org/10.1016/j.celrep.2020.107625
Flowers, B. M., Xu, H., Mulligan, A. S., Hanson, K. J., Seoane, J. A., Vogel, H., et al. (2021). Cell of origin influences pancreatic cancer subtype. Cancer Discovery, 11(3), 660–677. https://doi.org/10.1158/2159-8290.Cd-20-0633
Andricovich, J., Perkail, S., Kai, Y., Casasanta, N., Peng, W., & Tzatsos, A. (2018). Loss of KDM6A activates super-enhancers to induce gender-specific squamous-like pancreatic cancer and confers sensitivity to BET inhibitors. Cancer Cell, 33(3), 512-526.e518. https://doi.org/10.1016/j.ccell.2018.02.003
Sherman, M. H., Yu, R. T., Tseng, T. W., Sousa, C. M., Liu, S., Truitt, M. L., et al. (2017). Stromal cues regulate the pancreatic cancer epigenome and metabolome. Proc Natl Acad Sci U S A, 114(5), 1129–1134. https://doi.org/10.1073/pnas.1620164114
Carrer, A., Trefely, S., Zhao, S., Campbell, S. L., Norgard, R. J., Schultz, K. C., et al. (2019). Acetyl-CoA metabolism supports multistep pancreatic tumorigenesis. Cancer Discovery, 9(3), 416–435. https://doi.org/10.1158/2159-8290.Cd-18-0567
Bulusu, V., Tumanov, S., Michalopoulou, E., van den Broek, N. J., MacKay, G., Nixon, C., et al. (2017). Acetate recapturing by nuclear acetyl-CoA synthetase 2 prevents loss of histone acetylation during oxygen and serum limitation. Cell Reports, 18(3), 647–658. https://doi.org/10.1016/j.celrep.2016.12.055
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F., & Kroemer, G. (2015). Acetyl coenzyme A: A central metabolite and second messenger. Cell Metabolism, 21(6), 805–821. https://doi.org/10.1016/j.cmet.2015.05.014
Shi, Y., Gao, W., Lytle, N. K., Huang, P., Yuan, X., Dann, A. M., et al. (2019). Targeting LIF-mediated paracrine interaction for pancreatic cancer therapy and monitoring. Nature, 569(7754), 131–135. https://doi.org/10.1038/s41586-019-1130-6
Potapova, I. A., El-Maghrabi, M. R., Doronin, S. V., & Benjamin, W. B. (2000). Phosphorylation of recombinant human ATP:citrate lyase by cAMP-dependent protein kinase abolishes homotropic allosteric regulation of the enzyme by citrate and increases the enzyme activity. Allosteric activation of ATP:citrate lyase by phosphorylated sugars. Biochemistry, 39(5), 1169–1179. https://doi.org/10.1021/bi992159y
Wellen, K. E., Hatzivassiliou, G., Sachdeva, U. M., Bui, T. V., Cross, J. R., & Thompson, C. B. (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science, 324(5930), 1076–1080. https://doi.org/10.1126/science.1164097
Lee, J. V., Carrer, A., Shah, S., Snyder, N. W., Wei, S., Venneti, S., et al. (2014). Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metabolism, 20(2), 306–319. https://doi.org/10.1016/j.cmet.2014.06.004
Zhao, S., Torres, A., Henry, R. A., Trefely, S., Wallace, M., Lee, J. V., et al. (2016). ATP-citrate lyase controls a glucose-to-acetate metabolic switch. Cell Reports, 17(4), 1037–1052. https://doi.org/10.1016/j.celrep.2016.09.069
Ying, H., Kimmelman, A. C., Lyssiotis, C. A., Hua, S., Chu, G. C., Fletcher-Sananikone, E., et al. (2012). Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell, 149(3), 656–670. https://doi.org/10.1016/j.cell.2012.01.058
Amendola, C. R., Mahaffey, J. P., Parker, S. J., Ahearn, I. M., Chen, W. C., Zhou, M., et al. (2019). KRAS4A directly regulates hexokinase 1. Nature, 576(7787), 482–486. https://doi.org/10.1038/s41586-019-1832-9
Son, J., Lyssiotis, C. A., Ying, H., Wang, X., Hua, S., Ligorio, M., et al. (2013). Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature, 496(7443), 101–105. https://doi.org/10.1038/nature12040
Sousa, C. M., Biancur, D. E., Wang, X., Halbrook, C. J., Sherman, M. H., Zhang, L., et al. (2016). Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature, 536(7617), 479–483. https://doi.org/10.1038/nature19084
Commisso, C., Davidson, S. M., Soydaner-Azeloglu, R. G., Parker, S. J., Kamphorst, J. J., Hackett, S., et al. (2013). Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature, 497(7451), 633–637. https://doi.org/10.1038/nature12138
Olivares, O., Mayers, J. R., Gouirand, V., Torrence, M. E., Gicquel, T., Borge, L., et al. (2017). Collagen-derived proline promotes pancreatic ductal adenocarcinoma cell survival under nutrient limited conditions. Nature Communications, 8, 16031. https://doi.org/10.1038/ncomms16031
Kim, P. K., Halbrook, C. J., Kerk, S. A., Radyk, M., Wisner, S., Kremer, D. M., et al. (2021). Hyaluronic acid fuels pancreatic cancer cell growth. Elife, 10, e62645. https://doi.org/10.7554/eLife.62645
Kottakis, F., Nicolay, B. N., Roumane, A., Karnik, R., Gu, H., Nagle, J. M., et al. (2016). LKB1 loss links serine metabolism to DNA methylation and tumorigenesis. Nature, 539(7629), 390–395. https://doi.org/10.1038/nature20132
Chini, C. C., Guerrico, A. M., Nin, V., Camacho-Pereira, J., Escande, C., Barbosa, M. T., et al. (2014). Targeting of NAD metabolism in pancreatic cancer cells: Potential novel therapy for pancreatic tumors. Clinical Cancer Research, 20(1), 120–130. https://doi.org/10.1158/1078-0432.Ccr-13-0150
Halbrook, C. J., Pontious, C., Kovalenko, I., Lapienyte, L., Dreyer, S., Lee, H. J., et al. (2019). Macrophage-released pyrimidines inhibit gemcitabine therapy in pancreatic cancer. Cell Metabolism, 29(6), 1390-1399.e1396. https://doi.org/10.1016/j.cmet.2019.02.001
Badgley, M. A., Kremer, D. M., Maurer, H. C., DelGiorno, K. E., Lee, H. J., Purohit, V., et al. (2020). Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science, 368(6486), 85–89. https://doi.org/10.1126/science.aaw9872
Mukhopadhyay, S., Biancur, D. E., Parker, S. J., Yamamoto, K., Banh, R. S., Paulo, J. A., et al. (2021). Autophagy is required for proper cysteine homeostasis in pancreatic cancer through regulation of SLC7A11. Proceedings of the National Academy of Sciences of the United States of America, 118(6), e2021475118. https://doi.org/10.1073/pnas.2021475118
Santana-Codina, N., Roeth, A. A., Zhang, Y., Yang, A., Mashadova, O., Asara, J. M., et al. (2018). Oncogenic KRAS supports pancreatic cancer through regulation of nucleotide synthesis. Nature Communications, 9(1), 4945. https://doi.org/10.1038/s41467-018-07472-8
Datta, R., Sivanand, S., Lau, A. N., Florek, L. V., Barbeau, A. M., Wyckoff, J., et al. (2022). Interactions with stromal cells promote a more oxidized cancer cell redox state in pancreatic tumors. Sci Adv, 8(3), eabg6383. https://doi.org/10.1126/sciadv.abg6383
Akakura, N., Kobayashi, M., Horiuchi, I., Suzuki, A., Wang, J., Chen, J., et al. (2001). Constitutive expression of hypoxia-inducible factor-1alpha renders pancreatic cancer cells resistant to apoptosis induced by hypoxia and nutrient deprivation. Cancer Research, 61(17), 6548–6554.
Schug, Z. T., Peck, B., Jones, D. T., Zhang, Q., Grosskurth, S., Alam, I. S., et al. (2015). Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell, 27(1), 57–71. https://doi.org/10.1016/j.ccell.2014.12.002
Comerford, S. A., Huang, Z., Du, X., Wang, Y., Cai, L., Witkiewicz, A. K., et al. (2014). Acetate dependence of tumors. Cell, 159(7), 1591–1602. https://doi.org/10.1016/j.cell.2014.11.020
Mews, P., Donahue, G., Drake, A. M., Luczak, V., Abel, T., & Berger, S. L. (2017). Acetyl-CoA synthetase regulates histone acetylation and hippocampal memory. Nature, 546(7658), 381–386. https://doi.org/10.1038/nature22405
Thienpont, B., Steinbacher, J., Zhao, H., D’Anna, F., Kuchnio, A., Ploumakis, A., et al. (2016). Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature, 537(7618), 63–68. https://doi.org/10.1038/nature19081
Chakraborty, A. A., Laukka, T., Myllykoski, M., Ringel, A. E., Booker, M. A., Tolstorukov, M. Y., et al. (2019). Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science, 363(6432), 1217–1222. https://doi.org/10.1126/science.aaw1026
Laukka, T., Mariani, C. J., Ihantola, T., Cao, J. Z., Hokkanen, J., Kaelin, W. G., Jr., et al. (2016). Fumarate and succinate regulate expression of hypoxia-inducible genes via TET enzymes. Journal of Biological Chemistry, 291(8), 4256–4265. https://doi.org/10.1074/jbc.M115.688762
Letouzé, E., Martinelli, C., Loriot, C., Burnichon, N., Abermil, N., Ottolenghi, C., et al. (2013). SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell, 23(6), 739–752. https://doi.org/10.1016/j.ccr.2013.04.018
Xiao, M., Yang, H., Xu, W., Ma, S., Lin, H., Zhu, H., et al. (2012). Inhibition of α-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes & Development, 26(12), 1326–1338. https://doi.org/10.1101/gad.191056.112
Sciacovelli, M., Gonçalves, E., Johnson, T. I., Zecchini, V. R., da Costa, A. S., Gaude, E., et al. (2016). Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature, 537(7621), 544–547. https://doi.org/10.1038/nature19353
Batie, M., Frost, J., Frost, M., Wilson, J. W., Schofield, P., & Rocha, S. (2019). Hypoxia induces rapid changes to histone methylation and reprograms chromatin. Science, 363(6432), 1222–1226. https://doi.org/10.1126/science.aau5870
Fujikura, K., Alruwaii, Z. I., Haffner, M. C., Trujillo, M. A., Roberts, N. J., Hong, S. M., et al. (2021). Downregulation of 5-hydroxymethylcytosine is an early event in pancreatic tumorigenesis. The Journal of Pathology, 254(3), 279–288. https://doi.org/10.1002/path.5682
Oldham, W. M., Clish, C. B., Yang, Y., & Loscalzo, J. (2015). Hypoxia-mediated increases in L-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metabolism, 22(2), 291–303. https://doi.org/10.1016/j.cmet.2015.06.021
Intlekofer, A. M., Dematteo, R. G., Venneti, S., Finley, L. W., Lu, C., Judkins, A. R., et al. (2015). Hypoxia induces production of L-2-hydroxyglutarate. Cell Metabolism, 22(2), 304–311. https://doi.org/10.1016/j.cmet.2015.06.023
Gupta, V. K., Sharma, N. S., Durden, B., Garrido, V. T., Kesh, K., Edwards, D., et al. (2021). Hypoxia-driven oncometabolite L-2HG maintains stemness-differentiation balance and facilitates immune evasion in pancreatic cancer. Cancer Research, 81(15), 4001–4013. https://doi.org/10.1158/0008-5472.Can-20-2562
Hayashi, A., Fan, J., Chen, R., Ho, Y. J., Makohon-Moore, A. P., Lecomte, N., et al. (2020). A unifying paradigm for transcriptional heterogeneity and squamous features in pancreatic ductal adenocarcinoma. Nat Cancer, 1(1), 59–74. https://doi.org/10.1038/s43018-019-0010-1
Waddell, N., Pajic, M., Patch, A. M., Chang, D. K., Kassahn, K. S., Bailey, P., et al. (2015). Whole genomes redefine the mutational landscape of pancreatic cancer. Nature, 518(7540), 495–501. https://doi.org/10.1038/nature14169
Luzzi, K. J., MacDonald, I. C., Schmidt, E. E., Kerkvliet, N., Morris, V. L., Chambers, A. F., et al. (1998). Multistep nature of metastatic inefficiency: Dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. American Journal of Pathology, 153(3), 865–873. https://doi.org/10.1016/s0002-9440(10)65628-3
Massagué, J., & Obenauf, A. C. (2016). Metastatic colonization by circulating tumour cells. Nature, 529(7586), 298–306. https://doi.org/10.1038/nature17038
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz, L. A., Jr., & Kinzler, K. W. (2013). Cancer genome landscapes. Science, 339(6127), 1546–1558. https://doi.org/10.1126/science.1235122
García-Jiménez, C., & Goding, C. R. (2019). Starvation and pseudo-starvation as drivers of cancer metastasis through translation reprogramming. Cell Metabolism, 29(2), 254–267. https://doi.org/10.1016/j.cmet.2018.11.018
Whittle, M. C., Izeradjene, K., Rani, P. G., Feng, L., Carlson, M. A., DelGiorno, K. E., et al. (2015). RUNX3 controls a metastatic switch in pancreatic ductal adenocarcinoma. Cell, 161(6), 1345–1360. https://doi.org/10.1016/j.cell.2015.04.048
Halbrook, C. J., Thurston, G., Boyer, S., Anaraki, C., Jiménez, J. A., McCarthy, A., et al. (2022). Differential integrated stress response and asparagine production drive symbiosis and therapy resistance of pancreatic adenocarcinoma cells. Nat Cancer, 3(11), 1386–1403. https://doi.org/10.1038/s43018-022-00463-1
Costa-Silva, B., Aiello, N. M., Ocean, A. J., Singh, S., Zhang, H., Thakur, B. K., et al. (2015). Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nature Cell Biology, 17(6), 816–826. https://doi.org/10.1038/ncb3169
Lee, J. W., Stone, M. L., Porrett, P. M., Thomas, S. K., Komar, C. A., Li, J. H., et al. (2019). Hepatocytes direct the formation of a pro-metastatic niche in the liver. Nature, 567(7747), 249–252. https://doi.org/10.1038/s41586-019-1004-y
Pommier, A., Anaparthy, N., Memos, N., Kelley, Z. L., Gouronnec, A., Yan, R., et al. (2018). Unresolved endoplasmic reticulum stress engenders immune-resistant, latent pancreatic cancer metastases. Science, 360(6394), eaao4908. https://doi.org/10.1126/science.aao4908
Walter, P., & Ron, D. (2011). The unfolded protein response: From stress pathway to homeostatic regulation. Science, 334(6059), 1081–1086. https://doi.org/10.1126/science.1209038
Chiou, S. H., Risca, V. I., Wang, G. X., Yang, D., Grüner, B. M., Kathiria, A. S., et al. (2017). BLIMP1 induces transient metastatic heterogeneity in pancreatic cancer. Cancer Discovery, 7(10), 1184–1199. https://doi.org/10.1158/2159-8290.Cd-17-0250
Chang, Q., Jurisica, I., Do, T., & Hedley, D. W. (2011). Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Research, 71(8), 3110–3120. https://doi.org/10.1158/0008-5472.Can-10-4049
Recouvreux, M. V., Moldenhauer, M. R., Galenkamp, K. M. O., Jung, M., James, B., Zhang, Y., et al. (2020). Glutamine depletion regulates Slug to promote EMT and metastasis in pancreatic cancer. The Journal of Experimental Medicine, 217(9), e20200388. https://doi.org/10.1084/jem.20200388
Jian, Z., Cheng, T., Zhang, Z., Raulefs, S., Shi, K., Steiger, K., et al. (2018). Glycemic variability promotes both local invasion and metastatic colonization by pancreatic ductal adenocarcinoma. Cellular and Molecular Gastroenterology and Hepatology, 6(4), 429–449. https://doi.org/10.1016/j.jcmgh.2018.07.003
Dauer, P., Sharma, N. S., Gupta, V. K., Durden, B., Hadad, R., Banerjee, S., et al. (2019). ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness.” Cell Death & Disease, 10(2), 132. https://doi.org/10.1038/s41419-019-1408-5
Carstens, J. L., Yang, S., Correa de Sampaio, P., Zheng, X., Barua, S., McAndrews, K. M., et al. (2021). Stabilized epithelial phenotype of cancer cells in primary tumors leads to increased colonization of liver metastasis in pancreatic cancer. Cell Rep, 35(2), 108990. https://doi.org/10.1016/j.celrep.2021.108990
Rhim, A. D., Mirek, E. T., Aiello, N. M., Maitra, A., Bailey, J. M., McAllister, F., et al. (2012). EMT and dissemination precede pancreatic tumor formation. Cell, 148(1–2), 349–361. https://doi.org/10.1016/j.cell.2011.11.025
Simeonov, K. P., Byrns, C. N., Clark, M. L., Norgard, R. J., Martin, B., Stanger, B. Z., et al. (2021). Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states. Cancer Cell, 39(8), 1150-1162.e1159. https://doi.org/10.1016/j.ccell.2021.05.005
Krauß, L., Urban, B. C., Hastreiter, S., Schneider, C., Wenzel, P., Hassan, Z., et al. (2022). HDAC2 facilitates pancreatic cancer metastasis. Cancer Research, 82(4), 695–707. https://doi.org/10.1158/0008-5472.Can-20-3209
Aiello, N. M., Maddipati, R., Norgard, R. J., Balli, D., Li, J., Yuan, S., et al. (2018). EMT subtype influences epithelial plasticity and mode of cell migration. Developmental Cell, 45(6), 681-695.e684. https://doi.org/10.1016/j.devcel.2018.05.027
McDonald, O. G., Wu, H., Timp, W., Doi, A., & Feinberg, A. P. (2011). Genome-scale epigenetic reprogramming during epithelial-to-mesenchymal transition. Nature Structural & Molecular Biology, 18(8), 867–874. https://doi.org/10.1038/nsmb.2084
Yuan, S., Natesan, R., Sanchez-Rivera, F. J., Li, J., Bhanu, N. V., Yamazoe, T., et al. (2020). Global regulation of the histone mark H3K36me2 underlies epithelial plasticity and metastatic progression. Cancer Discovery, 10(6), 854–871. https://doi.org/10.1158/2159-8290.Cd-19-1299
Jia, S., Noma, K., & Grewal, S. I. (2004). RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science, 304(5679), 1971–1976. https://doi.org/10.1126/science.1099035
Tasdogan, A., Ubellacker, J. M., & Morrison, S. J. (2021). Redox regulation in cancer cells during metastasis. Cancer Discovery, 11(11), 2682–2692. https://doi.org/10.1158/2159-8290.Cd-21-0558
Zhang, Y., Xu, Y., Lu, W., Li, J., Yu, S., Brown, E. J., et al. (2022). G6PD-mediated increase in de novo NADP(+) biosynthesis promotes antioxidant defense and tumor metastasis. Sci Adv, 8(29), eabo0404. https://doi.org/10.1126/sciadv.abo0404
Bechard, M. E., Smalling, R., Hayashi, A., Zhong, Y., Word, A. E., Campbell, S. L., et al. (2020). Pancreatic cancers suppress negative feedback of glucose transport to reprogram chromatin for metastasis. Nature Communications, 11(1), 4055. https://doi.org/10.1038/s41467-020-17839-5
Torphy, R. J., Wang, Z., True-Yasaki, A., Volmar, K. E., Rashid, N., Yeh, B., et al. (2018). Stromal content is correlated with tissue site, contrast retention, and survival in pancreatic adenocarcinoma. JCO Precision Oncology, 2018, PO.17.00121. https://doi.org/10.1200/po.17.00121
Yachida, S., Jones, S., Bozic, I., Antal, T., Leary, R., Fu, B., et al. (2010). Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature, 467(7319), 1114–1117. https://doi.org/10.1038/nature09515
Makohon-Moore, A. P., Zhang, M., Reiter, J. G., Bozic, I., Allen, B., Kundu, D., et al. (2017). Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nature Genetics, 49(3), 358–366. https://doi.org/10.1038/ng.3764
Maddipati, R., & Stanger, B. Z. (2015). Pancreatic cancer metastases harbor evidence of polyclonality. Cancer Discovery, 5(10), 1086–1097. https://doi.org/10.1158/2159-8290.Cd-15-0120
Iacobuzio-Donahue, C. A., Litchfield, K., & Swanton, C. (2020). Intratumor heterogeneity reflects clinical disease course. Nature Cancer, 1(1), 3–6. https://doi.org/10.1038/s43018-019-0002-1
McDonald, O. G., Li, X., Saunders, T., Tryggvadottir, R., Mentch, S. J., Warmoes, M. O., et al. (2017). Epigenomic reprogramming during pancreatic cancer progression links anabolic glucose metabolism to distant metastasis. Nature Genetics, 49(3), 367–376. https://doi.org/10.1038/ng.3753
Roe, J. S., Hwang, C. I., Somerville, T. D. D., Milazzo, J. P., Lee, E. J., Da Silva, B., et al. (2017). Enhancer reprogramming promotes pancreatic cancer metastasis. Cell, 170(5), 875-888.e820. https://doi.org/10.1016/j.cell.2017.07.007
Connor, A. A., Denroche, R. E., Jang, G. H., Lemire, M., Zhang, A., Chan-Seng-Yue, M., et al. (2019). Integration of genomic and transcriptional features in pancreatic cancer reveals increased cell cycle progression in metastases. Cancer Cell, 35(2), 267-282.e267. https://doi.org/10.1016/j.ccell.2018.12.010
Bechard, M. E., Word, A. E., Tran, A. V., Liu, X., Locasale, J. W., & McDonald, O. G. (2018). Pentose conversions support the tumorigenesis of pancreatic cancer distant metastases. Oncogene, 37(38), 5248–5256. https://doi.org/10.1038/s41388-018-0346-5
Smalling, R. V., Bechard, M. E., Duryea, J., Kingsley, P. J., Roberts, E. R., Marnett, L. J., et al. (2022). Aminopyridine analogs selectively target metastatic pancreatic cancer. Oncogene, 41(10), 1518–1525. https://doi.org/10.1038/s41388-022-02183-3
Reiter, J. G., Makohon-Moore, A. P., Gerold, J. M., Heyde, A., Attiyeh, M. A., Kohutek, Z. A., et al. (2018). Minimal functional driver gene heterogeneity among untreated metastases. Science, 361(6406), 1033–1037. https://doi.org/10.1126/science.aat7171
Jiang, H., Torphy, R. J., Steiger, K., Hongo, H., Ritchie, A. J., Kriegsmann, M., et al. (2020). Pancreatic ductal adenocarcinoma progression is restrained by stromal matrix. The Journal of Clinical Investigation, 130(9), 4704–4709. https://doi.org/10.1172/jci136760
Bergers, G., & Fendt, S. M. (2021). The metabolism of cancer cells during metastasis. Nature Reviews Cancer, 21(3), 162–180. https://doi.org/10.1038/s41568-020-00320-2
Iacobuzio-Donahue, C. A., Fu, B., Yachida, S., Luo, M., Abe, H., Henderson, C. M., et al. (2009). DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. Journal of Clinical Oncology, 27(11), 1806–1813. https://doi.org/10.1200/jco.2008.17.7188
Rhim, A. D., Oberstein, P. E., Thomas, D. H., Mirek, E. T., Palermo, C. F., Sastra, S. A., et al. (2014). Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell, 25(6), 735–747. https://doi.org/10.1016/j.ccr.2014.04.021
McDonald, O. G. (2020). Cancer metastasis: Selectable traits without genetic constraints. Mol Cell Oncol, 7(6), 1825910. https://doi.org/10.1080/23723556.2020.1825910
He, D., Feng, H., Sundberg, B., Yang, J., Powers, J., Christian, A. H., et al. (2022). Methionine oxidation activates pyruvate kinase M2 to promote pancreatic cancer metastasis. Molecular Cell, 82(16), 3045-3060.e3011. https://doi.org/10.1016/j.molcel.2022.06.005
Salas, J. R., & Clark, P. M. (2022). Signaling pathways that drive (18)F-FDG accumulation in cancer. Journal of Nuclear Medicine, 63(5), 659–663. https://doi.org/10.2967/jnumed.121.262609
Ghergurovich, J. M., Esposito, M., Chen, Z., Wang, J. Z., Bhatt, V., Lan, T., et al. (2020). Glucose-6-phosphate dehydrogenase is not essential for K-Ras-driven tumor growth or metastasis. Cancer Research, 80(18), 3820–3829. https://doi.org/10.1158/0008-5472.Can-19-2486
Shan, C., Elf, S., Ji, Q., Kang, H. B., Zhou, L., Hitosugi, T., et al. (2014). Lysine acetylation activates 6-phosphogluconate dehydrogenase to promote tumor growth. Molecular Cell, 55(4), 552–565. https://doi.org/10.1016/j.molcel.2014.06.020
Zhang, Y., Xu, Y., Lu, W., Ghergurovich, J. M., Guo, L., Blair, I. A., et al. (2021). Upregulation of antioxidant capacity and nucleotide precursor availability suffices for oncogenic transformation. Cell Metabolism, 33(1), 94-109.e108. https://doi.org/10.1016/j.cmet.2020.10.002
Billin, A. N., Eilers, A. L., Coulter, K. L., Logan, J. S., & Ayer, D. E. (2000). MondoA, a novel basic helix-loop-helix-leucine zipper transcriptional activator that constitutes a positive branch of a max-like network. Molecular and Cellular Biology, 20(23), 8845–8854. https://doi.org/10.1128/mcb.20.23.8845-8854.2000
Stoltzman, C. A., Peterson, C. W., Breen, K. T., Muoio, D. M., Billin, A. N., & Ayer, D. E. (2008). Glucose sensing by MondoA: Mlx complexes: A role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci U S A, 105(19), 6912–6917. https://doi.org/10.1073/pnas.0712199105
Peterson, C. W., Stoltzman, C. A., Sighinolfi, M. P., Han, K. S., & Ayer, D. E. (2010). Glucose controls nuclear accumulation, promoter binding, and transcriptional activity of the MondoA-Mlx heterodimer. Molecular and Cellular Biology, 30(12), 2887–2895. https://doi.org/10.1128/mcb.01613-09
Stoltzman, C. A., Kaadige, M. R., Peterson, C. W., & Ayer, D. E. (2011). MondoA senses non-glucose sugars: Regulation of thioredoxin-interacting protein (TXNIP) and the hexose transport curb. Journal of Biological Chemistry, 286(44), 38027–38034. https://doi.org/10.1074/jbc.M111.275503
Wu, N., Zheng, B., Shaywitz, A., Dagon, Y., Tower, C., Bellinger, G., et al. (2013). AMPK-dependent degradation of TXNIP upon energy stress leads to enhanced glucose uptake via GLUT1. Molecular Cell, 49(6), 1167–1175. https://doi.org/10.1016/j.molcel.2013.01.035
Selak, M. A., Armour, S. M., MacKenzie, E. D., Boulahbel, H., Watson, D. G., Mansfield, K. D., et al. (2005). Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell, 7(1), 77–85. https://doi.org/10.1016/j.ccr.2004.11.022
Maddalena, M., Mallel, G., Nataraj, N. B., Shreberk-Shaked, M., Hassin, O., Mukherjee, S., et al. (2021). TP53 missense mutations in PDAC are associated with enhanced fibrosis and an immunosuppressive microenvironment. Proceedings of the National Academy of Sciences of the United States of America, 118(23), e2025631118. https://doi.org/10.1073/pnas.2025631118
Siolas, D., Vucic, E., Kurz, E., Hajdu, C., & Bar-Sagi, D. (2021). Gain-of-function p53(R172H) mutation drives accumulation of neutrophils in pancreatic tumors, promoting resistance to immunotherapy. Cell Rep, 36(8), 109578. https://doi.org/10.1016/j.celrep.2021.109578
Martin, T. D., Patel, R. S., Cook, D. R., Choi, M. Y., Patil, A., Liang, A. C., et al. (2021). The adaptive immune system is a major driver of selection for tumor suppressor gene inactivation. Science, 373(6561), 1327–1335. https://doi.org/10.1126/science.abg5784
Su, X., Wellen, K. E., & Rabinowitz, J. D. (2016). Metabolic control of methylation and acetylation. Current Opinion in Chemical Biology, 30, 52–60. https://doi.org/10.1016/j.cbpa.2015.10.030
Mentch, S. J., Mehrmohamadi, M., Huang, L., Liu, X., Gupta, D., Mattocks, D., et al. (2015). Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metabolism, 22(5), 861–873. https://doi.org/10.1016/j.cmet.2015.08.024
Yang, S., Wang, X., Contino, G., Liesa, M., Sahin, E., Ying, H., et al. (2011). Pancreatic cancers require autophagy for tumor growth. Genes & Development, 25(7), 717–729. https://doi.org/10.1101/gad.2016111
Haws, S. A., Yu, D., Ye, C., Wille, C. K., Nguyen, L. C., Krautkramer, K. A., et al. (2020). Methyl-metabolite depletion elicits adaptive responses to support heterochromatin stability and epigenetic persistence. Molecular Cell, 78(2), 210-223.e218. https://doi.org/10.1016/j.molcel.2020.03.004
Ye, C., Sutter, B. M., Wang, Y., Kuang, Z., & Tu, B. P. (2017). A metabolic function for phospholipid and histone methylation. Molecular Cell, 66(2), 180-193.e188. https://doi.org/10.1016/j.molcel.2017.02.026
Tiwari, A., Tashiro, K., Dixit, A., Soni, A., Vogel, K., Hall, B., et al. (2020). Loss of HIF1A from pancreatic cancer cells increases expression of PPP1R1B and degradation of p53 to promote invasion and metastasis. Gastroenterology, 159(5), 1882-1897.e1885. https://doi.org/10.1053/j.gastro.2020.07.046
Sullivan, W. J., Mullen, P. J., Schmid, E. W., Flores, A., Momcilovic, M., Sharpley, M. S., et al. (2018). Extracellular matrix remodeling regulates glucose metabolism through TXNIP destabilization. Cell, 175(1), 117-132.e121. https://doi.org/10.1016/j.cell.2018.08.017
Wellen, K. E., & Snyder, N. W. (2019). Should we consider subcellular compartmentalization of metabolites, and if so, how do we measure them? Current Opinion in Clinical Nutrition and Metabolic Care, 22(5), 347–354. https://doi.org/10.1097/mco.0000000000000580
Sivanand, S., Rhoades, S., Jiang, Q., Lee, J. V., Benci, J., Zhang, J., et al. (2017). Nuclear acetyl-CoA production by ACLY promotes homologous recombination. Molecular Cell, 67(2), 252-265.e256. https://doi.org/10.1016/j.molcel.2017.06.008
Sulkowski, P. L., Oeck, S., Dow, J., Economos, N. G., Mirfakhraie, L., Liu, Y., et al. (2020). Oncometabolites suppress DNA repair by disrupting local chromatin signalling. Nature, 582(7813), 586–591. https://doi.org/10.1038/s41586-020-2363-0
Dougan, S. K. (2017). The pancreatic cancer microenvironment. Cancer Journal, 23(6), 321–325. https://doi.org/10.1097/ppo.0000000000000288
Li, N., Grivennikov, S. I., & Karin, M. (2011). The unholy trinity: Inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell, 19(4), 429–431. https://doi.org/10.1016/j.ccr.2011.03.018
Whatcott, C. J., Diep, C. H., Jiang, P., Watanabe, A., LoBello, J., Sima, C., et al. (2015). Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clinical Cancer Research, 21(15), 3561–3568. https://doi.org/10.1158/1078-0432.Ccr-14-1051
Aiello, N. M., Bajor, D. L., Norgard, R. J., Sahmoud, A., Bhagwat, N., Pham, M. N., et al. (2016). Metastatic progression is associated with dynamic changes in the local microenvironment. Nature Communications, 7, 12819. https://doi.org/10.1038/ncomms12819
Bhagat, T. D., Von Ahrens, D., Dawlaty, M., Zou, Y., Baddour, J., Achreja, A., et al. (2019). Lactate-mediated epigenetic reprogramming regulates formation of human pancreatic cancer-associated fibroblasts. Elife, 8, e50663. https://doi.org/10.7554/eLife.50663.
Schwörer, S., Vardhana, S. A., & Thompson, C. B. (2019). Cancer metabolism drives a stromal regenerative response. Cell Metabolism, 29(3), 576–591. https://doi.org/10.1016/j.cmet.2019.01.015
Faubert, B., Solmonson, A., & DeBerardinis, R. J. (2020). Metabolic reprogramming and cancer progression. Science, 368(6487), https://doi.org/10.1126/science.aaw5473.
Steele, C. W., Karim, S. A., Leach, J. D. G., Bailey, P., Upstill-Goddard, R., Rishi, L., et al. (2016). CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell, 29(6), 832–845. https://doi.org/10.1016/j.ccell.2016.04.014
Funding
This work is supported by National Institutes of Health grant R01 CA222594 (OGM).
Author information
Authors and Affiliations
Contributions
AJFT, JD, and OGM wrote the text and assembled the figures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Torres, A.J.F., Duryea, J. & McDonald, O.G. Pancreatic cancer epigenetics: adaptive metabolism reprograms starving primary tumors for widespread metastatic outgrowth. Cancer Metastasis Rev 42, 389–407 (2023). https://doi.org/10.1007/s10555-023-10116-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-023-10116-z