Abstract
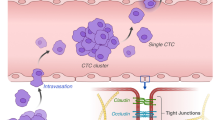
Metastasis is a multistep process that accounts for the majority of cancer-related death. By the end of metastasize dissemination, circulating tumor cells (CTC) need to extravasate the blood vessels at metastatic sites to form new colonization. Although cancer cell extravasation is a crucial step in cancer metastasis, it has not been successfully targeted by current anti-metastasis strategies due to the lack of a thorough understanding of the molecular mechanisms that regulate this process. This review focuses on recent progress in cancer extravasation visualization techniques, including the development of both in vitro and in vivo cancer extravasation models, that shed light on the underlying mechanisms. Specifically, multiple cancer extravasation stages, such as the adhesion to the endothelium and transendothelial migration, are successfully probed using these technologies. Moreover, the roles of different cell adhesive molecules, chemokines, and growth factors, as well as the mechanical factors in these stages are well illustrated. Deeper understandings of cancer extravasation mechanisms offer us new opportunities to escalate the discovery of anti-extravasation drugs and therapies and improve the prognosis of cancer patients.



Similar content being viewed by others
References
Eccles, S. A., & Welch, D. R. (2007). Metastasis: recent discoveries and novel treatment strategies. The Lancet, 369(9574), 1742–1757.
Sahai, E. (2007). Illuminating the metastatic process. Nature Reviews Cancer, 7(10), 737–749.
Steeg, P. S. (2016). Targeting metastasis. Nature Reviews Cancer, 16(4), 201–218.
Reymond, N., d’Agua, B. B., & Ridley, A. J. (2013). Crossing the endothelial barrier during metastasis. Nature Reviews Cancer, 13(12), 858–870.
Strilic, B., & Offermanns, S. (2017). Intravascular survival and extravasation of tumor cells. Cancer Cell, 32(3), 282–293.
Chakrabarti, R., Hwang, J., Blanco, M. A., Wei, Y., Lukačišin, M., Romano, R.-A., et al. (2012). Elf5 inhibits the epithelial–mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nature Cell Biology, 14(11), 1212–1222.
Giampieri, S., Manning, C., Hooper, S., Jones, L., Hill, C. S., & Sahai, E. (2009). Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nature Cell Biology, 11(11), 1287–1296.
Xue, C., Wyckoff, J., Liang, F., Sidani, M., Violini, S., Tsai, K.-L., Zhang, Z. Y., Sahai, E., Condeelis, J., & Segall, J. E. (2006). Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Research, 66(1), 192–197.
Allen, T. A., Asad, D., Amu, E., Hensley, M. T., Cores, J., Vandergriff, A., et al. (2019). Circulating tumor cells exit circulation while maintaining multicellularity, augmenting metastatic potential. J Cell Sci, 132(17), jcs231563.
Bittner, K. R., Jiménez, J. M., & Peyton, S. R. (2020). Vascularized biomaterials to study cancer metastasis. Advanced Healthcare Materials, 9(8), 1901459.
Paku, S., Laszlo, V., Dezso, K., Nagy, P., Hoda, M. A., Klepetko, W., Renyi-Vamos, F., Timar, J., Reynolds, A. R., & Dome, B. (2017). The evidence for and against different modes of tumour cell extravasation in the lung: diapedesis, capillary destruction, necroptosis, and endothelialization. The Journal of Pathology, 241(4), 441–447.
Follain, G., Osmani, N., Azevedo, A. S., Allio, G., Mercier, L., Karreman, M. A., et al. (2018). Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev Cell, 45(1), 33–52. e12.
Strell, C., & Entschladen, F. (2008). Extravasation of leukocytes in comparison to tumor cells. Cell Commun Signal, 6(1), 10.
Cheng, K., Shen, D., Xie, Y., Cingolani, E., Malliaras, K., & Marbán, E. (2012). Brief report: mechanism of extravasation of infused stem cells. Stem Cells, 30(12), 2835–2842.
Allen, T. A., Gracieux, D., Talib, M., Tokarz, D. A., Hensley, M. T., Cores, J., Vandergriff, A., Tang, J., de Andrade, J. B. M., Dinh, P. U., Yoder, J. A., & Cheng, K. (2017). Angiopellosis as an alternative mechanism of cell extravasation. Stem Cells, 35(1), 170–180.
Chen, M. B., Whisler, J. A., Fröse, J., Yu, C., Shin, Y., & Kamm, R. D. (2017). On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nature Protocols, 12(5), 865–880.
Pan, C., Schoppe, O., Parra-Damas, A., Cai, R., Todorov, M. I., Gondi, G., et al. (2019). Deep learning reveals cancer metastasis and therapeutic antibody targeting in the entire body. Cell, 179(7), 1661–1676. e1619.
Peng, F., Setyawati, M. I., Tee, J. K., Ding, X., Wang, J., Nga, M. E., Ho, H. K., & Leong, D. T. (2019). Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nature Nanotechnology, 14(3), 279–286.
Kramer, R. H., & Nicolson, G. L. (1979). Interactions of tumor cells with vascular endothelial cell monolayers: a model for metastatic invasion. Proceedings of the National Academy of Sciences, 76(11), 5704–5708.
Nicolson, G. L. (1982). Metastatic tumor cell attachment and invasion assay utilizing vascular endothelial cell monolayers. Journal of Histochemistry & Cytochemistry, 30(3), 214–220.
Kramer, R. H., & Nicolson, G. L. (1981). Invasion of vascular endothelial cell monolayers and underlying matrix by metastatic human cancer cells. In H.G. Schweiger (Eds.), International Cell Biology 1980–1981 (pp. 794–799). Berlin, Heidelberg: Springer.
Kang, S.-A., Bajana, S., & Tanaka, T. (2016). In vitro flow adhesion assay for analyzing shear-resistant adhesion of metastatic cancer cells to endothelial cells. Bio-protocol, 6(4), e1731.
Spencer, A., Spruell, C., Nandi, S., Wong, M., Creixell, M., & Baker, A. B. (2016). A high-throughput mechanofluidic screening platform for investigating tumor cell adhesion during metastasis. Lab on a Chip, 16(1), 142–152.
Pouliot, N., Pearson, H. B., & Burrows, A. (2013). Investigating metastasis using in vitro platforms. In R. Jandial (Ed.), Madame Curie Bioscience Database Georgetown: Landes Bioscience.
Katt, M. E., Placone, A. L., Wong, A. D., Xu, Z. S., & Searson, P. C. (2016). In vitro tumor models: advantages, disadvantages, variables, and selecting the right platform. Frontiers in Bioengineering and Biotechnology, 4, 12.
Jeon, J., Zervantonakis, I., Chung, S., Kamm, R., & Charest, J. (2013). In vitro model of tumor cell extravasation. PLoS One, 8, e56910.
Ma, Y.-H. V., Middleton, K., You, L., & Sun, Y. (2018). A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsystems & Nanoengineering, 4(1), 1–13.
Mierke, C. T. (2011). Cancer cells regulate biomechanical properties of human microvascular endothelial cells. Journal of Biological Chemistry, 286(46), 40025–40037.
Li, Y.-H., & Zhu, C. (1999). A modified Boyden chamber assay for tumor cell transendothelial migration in vitro. Clinical & Experimental Metastasis, 17(5), 423–429.
Laferrière, J., Houle, F., Taher, M. M., Valerie, K., & Huot, J. (2001). Transendothelial migration of colon carcinoma cells requires expression of E-selectin by endothelial cells and activation of stress-activated protein kinase-2 (SAPK2/p38) in the tumor cells. Journal of Biological Chemistry, 276(36), 33762–33772.
Lee, W., Choong, L., Jin, T., Mon, N., Chong, S., Liew, C., et al. (2017). TRPV4 plays a role in breast cancer cell migration via Ca 2+-dependent activation of AKT and downregulation of E-cadherin cell cortex protein. Oncogenesis, 6(5), e338–e338.
Pignatelli, J., Goswami, S., Jones, J. G., Rohan, T. E., Pieri, E., Chen, X., et al. (2014). Invasive breast carcinoma cells from patients exhibit MenaINV-and macrophage-dependent transendothelial migration. Science Signaling, 7(353), ra112.
Orellana, R., Kato, S., Erices, R., Bravo, M. L., Gonzalez, P., Oliva, B., Cubillos, S., Valdivia, A., Ibañez, C., Brañes, J., Barriga, M. I., Bravo, E., Alonso, C., Bustamente, E., Castellon, E., Hidalgo, P., Trigo, C., Panes, O., Pereira, J., Mezzano, D., Cuello, M. A., & Owen, G. I. (2015). Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells. BMC Cancer, 15(1), 290.
Carter, J. C., & Church, F. C. (2012). Mature breast adipocytes promote breast cancer cell motility. Experimental and Molecular Pathology, 92(3), 312–317.
Gassmann, P., Haier, J., Schlüter, K., Domikowsky, B., Wendel, C., Wiesner, U., et al. (2009). CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia (New York, NY), 11(7), 651.
Cao, Y., Hoeppner, L. H., Bach, S., Guangqi, E., Guo, Y., Wang, E., et al. (2013). Neuropilin-2 promotes extravasation and metastasis by interacting with endothelial α5 integrin. Cancer Research, 73(14), 4579–4590.
Chrobak, K. M., Potter, D. R., & Tien, J. (2006). Formation of perfused, functional microvascular tubes in vitro. Microvascular Research, 71(3), 185–196.
Miller, J. S., Stevens, K. R., Yang, M. T., Baker, B. M., Nguyen, D.-H. T., Cohen, D. M., Toro, E., Chen, A. A., Galie, P. A., Yu, X., Chaturvedi, R., Bhatia, S. N., & Chen, C. S. (2012). Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nature Materials, 11(9), 768–774.
Wang, X.-Y., Pei, Y., Xie, M., Jin, Z.-H., Xiao, Y.-S., Wang, Y., Zhang, L. N., Li, Y., & Huang, W. H. (2015). An artificial blood vessel implanted three-dimensional microsystem for modeling transvascular migration of tumor cells. Lab on a Chip, 15(4), 1178–1187.
Zheng, Y., Chen, J., Craven, M., Choi, N. W., Totorica, S., Diaz-Santana, A., Kermani, P., Hempstead, B., Fischbach-Teschl, C., Lopez, J. A., & Stroock, A. D. (2012). In vitro microvessels for the study of angiogenesis and thrombosis. Proceedings of the National Academy of Sciences, 109(24), 9342–9347.
Moya, M. L., Hsu, Y.-H., Lee, A. P., Hughes, C. C., & George, S. C. (2013). In vitro perfused human capillary networks. Tissue Engineering Part C: Methods, 19(9), 730–737.
Shirure, V. S., Bi, Y., Curtis, M. B., Lezia, A., Goedegebuure, M. M., Goedegebuure, S. P., Aft, R., Fields, R. C., & George, S. C. (2018). Tumor-on-a-chip platform to investigate progression and drug sensitivity in cell lines and patient-derived organoids. Lab on a Chip, 18(23), 3687–3702.
Chen, X., Aledia, A. S., Ghajar, C. M., Griffith, C. K., Putnam, A. J., Hughes, C. C., et al. (2009). Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Engineering Part A, 15(6), 1363–1371.
Chen, M. B., Whisler, J. A., Jeon, J. S., & Kamm, R. D. (2013). Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integrative Biology, 5(10), 1262–1271.
Paek, J., Park, S. E., Lu, Q., Park, K.-T., Cho, M., Oh, J. M., Kwon, K. W., Yi, Y. S., Song, J. W., Edelstein, H. I., Ishibashi, J., Yang, W., Myerson, J. W., Kiseleva, R. Y., Aprelev, P., Hood, E. D., Stambolian, D., Seale, P., Muzykantov, V. R., & Huh, D. (2019). Microphysiological engineering of self-assembled and Perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano, 13(7), 7627–7643.
Kim, Y., Williams, K. C., Gavin, C. T., Jardine, E., Chambers, A. F., & Leong, H. S. (2016). Quantification of cancer cell extravasation in vivo. Nature Protocols, 11(5), 937–948.
Heyder, C., Gloria-Maercker, E., Entschladen, F., Hatzmann, W., Niggemann, B., Zänker, K., & Dittmar, T. (2002). Realtime visualization of tumor cell/endothelial cell interactions during transmigration across the endothelial barrier. Journal of Cancer Research and Clinical Oncology, 128(10), 533–538.
Strilic, B., Yang, L., Albarrán-Juárez, J., Wachsmuth, L., Han, K., Müller, U. C., Pasparakis, M., & Offermanns, S. (2016). Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature, 536(7615), 215–218.
Shin, M. K., Kim, S. K., & Jung, H. (2011). Integration of intra-and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab on a Chip, 11(22), 3880–3887.
Cui, X., Guo, W., Sun, Y., Sun, B., Hu, S., Sun, D., & Lam, R. H. W. (2017). A microfluidic device for isolation and characterization of transendothelial migrating cancer cells. Biomicrofluidics, 11(1), 014105.
Song, J. W., Cavnar, S. P., Walker, A. C., Luker, K. E., Gupta, M., Tung, Y.-C., Luker, G. D., & Takayama, S. (2009). Microfluidic endothelium for studying the intravascular adhesion of metastatic breast cancer cells. PLoS One. https://doi.org/10.1371/journal.pone.0005756
Xu, H., Li, Z., Yu, Y., Sizdahkhani, S., Ho, W. S., Yin, F., Wang, L., Zhu, G., Zhang, M., Jiang, L., Zhuang, Z., & Qin, J. (2016). A dynamic in vivo-like organotypic blood-brain barrier model to probe metastatic brain tumors. Scientific Reports, 6, 36670.
Chen, M. B., Hajal, C., Benjamin, D. C., Yu, C., Azizgolshani, H., Hynes, R. O., et al. (2018). Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proceedings of the National Academy of Sciences, 115(27), 7022–7027.
Jeon, J. S., Bersini, S., Gilardi, M., Dubini, G., Charest, J. L., Moretti, M., & Kamm, R. D. (2015). Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proceedings of the National Academy of Sciences, 112(1), 214–219.
Coughlin, M. F., & Kamm, R. D. (2020). The use of microfluidic platforms to probe the mechanism of cancer cell extravasation. Advanced Healthcare Materials, 9(8), 1901410.
Kolesky, D. B., Homan, K. A., Skylar-Scott, M. A., & Lewis, J. A. (2016). Three-dimensional bioprinting of thick vascularized tissues. Proceedings of the National Academy of Sciences, 113(12), 3179–3184.
Hinton, T. J., Jallerat, Q., Palchesko, R. N., Park, J. H., Grodzicki, M. S., Shue, H.-J., Ramadan, M. H., Hudson, A. R., & Feinberg, A. W. (2015). Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Advances, 1(9), e1500758.
Entenberg, D., Voiculescu, S., Guo, P., Borriello, L., Wang, Y., Karagiannis, G. S., Jones, J., Baccay, F., Oktay, M., & Condeelis, J. (2018). A permanent window for the murine lung enables high-resolution imaging of cancer metastasis. Nature Methods, 15(1), 73–80.
Kienast, Y., Von Baumgarten, L., Fuhrmann, M., Klinkert, W. E., Goldbrunner, R., Herms, J., et al. (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nature Medicine, 16(1), 116.
Condeelis, J., & Segall, J. E. (2003). Intravital imaging of cell movement in tumours. Nature Reviews Cancer, 3(12), 921–930.
Cao, J., Zhu, B., Zheng, K., He, S., Meng, L., Song, J., et al. (2019). Recent progress in NIR-II contrast agent for biological imaging. Frontiers in Bioengineering and Biotechnology, 7. 487.
Smith, A. M., Mancini, M. C., & Nie, S. (2009). Second window for in vivo imaging. Nature Nanotechnology, 4(11), 710–711.
Welsher, K., Sherlock, S. P., & Dai, H. (2011). Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proceedings of the National Academy of Sciences, 108(22), 8943–8948.
Welsher, K., Liu, Z., Sherlock, S. P., Robinson, J. T., Chen, Z., Daranciang, D., & Dai, H. (2009). A route to brightly fluorescent carbon nanotubes for near-infrared imaging in mice. Nature Nanotechnology, 4(11), 773–780.
Hong, G., Lee, J. C., Robinson, J. T., Raaz, U., Xie, L., Huang, N. F., Cooke, J. P., & Dai, H. (2012). Multifunctional in vivo vascular imaging using near-infrared II fluorescence. Nature Medicine, 18(12), 1841–1846.
Hong, G., Robinson, J. T., Zhang, Y., Diao, S., Antaris, A. L., Wang, Q., & Dai, H. (2012). In vivo fluorescence imaging with Ag2S quantum dots in the second near-infrared region. Angewandte Chemie International Edition, 51(39), 9818–9821.
Tian, R., Ma, H., Zhu, S., Lau, J., Ma, R., Liu, Y., Lin, L., Chandra, S., Wang, S., Zhu, X., Deng, H., Niu, G., Zhang, M., Antaris, A. L., Hettie, K. S., Yang, B., Liang, Y., & Chen, X. (2020). Multiplexed NIR-II probes for lymph node-invaded cancer detection and imaging-guided surgery. Advanced Materials, 32(11), 1907365.
Antaris, A. L., Chen, H., Cheng, K., Sun, Y., Hong, G., Qu, C., Diao, S., Deng, Z., Hu, X., Zhang, B., Zhang, X., Yaghi, O. K., Alamparambil, Z. R., Hong, X., Cheng, Z., & Dai, H. (2016). A small-molecule dye for NIR-II imaging. Nature Materials, 15(2), 235–242.
Zhang, Y., Hong, G., Zhang, Y., Chen, G., Li, F., Dai, H., & Wang, Q. (2012). Ag2S quantum dot: a bright and biocompatible fluorescent nanoprobe in the second near-infrared window. ACS Nano, 6(5), 3695–3702.
Zhang, Y., Zhang, Y., Hong, G., He, W., Zhou, K., Yang, K., Li, F., Chen, G., Liu, Z., Dai, H., & Wang, Q. (2013). Biodistribution, pharmacokinetics and toxicology of Ag2S near-infrared quantum dots in mice. Biomaterials, 34(14), 3639–3646.
Flusberg, B. A., Cocker, E. D., Piyawattanametha, W., Jung, J. C., Cheung, E. L., & Schnitzer, M. J. (2005). Fiber-optic fluorescence imaging. Nature Methods, 2(12), 941–950.
Ritsma, L., Steller, E. J., Beerling, E., Loomans, C. J., Zomer, A., Gerlach, C., et al. (2012). Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Science Translational Medicine, 4(158), 158ra145.
Chambers, A. F., Groom, A. C., & MacDonald, I. C. (2002). Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer, 2(8), 563–572.
Yamauchi, K., Yang, M., Jiang, P., Xu, M., Yamamoto, N., Tsuchiya, H., Tomita, K., Moossa, A. R., Bouvet, M., & Hoffman, R. M. (2006). Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Research, 66(8), 4208–4214.
Cai, R., Pan, C., Ghasemigharagoz, A., Todorov, M. I., Förstera, B., Zhao, S., Bhatia, H. S., Parra-Damas, A., Mrowka, L., Theodorou, D., Rempfler, M., Xavier, A. L. R., Kress, B. T., Benakis, C., Steinke, H., Liebscher, S., Bechmann, I., Liesz, A., Menze, B., Kerschensteiner, M., Nedergaard, M., & Ertürk, A. (2019). Panoptic imaging of transparent mice reveals whole-body neuronal projections and skull–meninges connections. Nature Neuroscience, 22(2), 317–327.
Nowak-Sliwinska, P., Segura, T., & Iruela-Arispe, M. L. (2014). The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis, 17(4), 779–804.
Leong, H. S., Robertson, A. E., Stoletov, K., Leith, S. J., Chin, C. A., Chien, A. E., Hague, M. N., Ablack, A., Carmine-Simmen, K., McPherson, V. A., Postenka, C. O., Turley, E. A., Courtneidge, S. A., Chambers, A. F., & Lewis, J. D. (2014). Invadopodia are required for cancer cell extravasation and are a therapeutic target for metastasis. Cell Reports, 8(5), 1558–1570.
Stoletov, K., Kato, H., Zardouzian, E., Kelber, J., Yang, J., Shattil, S., & Klemke, R. (2010). Visualizing extravasation dynamics of metastatic tumor cells. Journal of Cell Science, 123(13), 2332–2341.
Kanada, M., Zhang, J., Yan, L., Sakurai, T., & Terakawa, S. (2014). Endothelial cell-initiated extravasation of cancer cells visualized in zebrafish. PeerJ, 2, e688.
Baeten, J. T., & de Jong, J. L. (2018). Genetic models of leukemia in zebrafish. Frontiers in Cell and Developmental Biology, 6, 115.
Isogai, S., Lawson, N. D., Torrealday, S., Horiguchi, M., & Weinstein, B. M. (2003). Angiogenic network formation in the developing vertebrate trunk. Development, 130(21), 5281–5290.
Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B., & Schilling, T. F. (1995). Stages of embryonic development of the zebrafish. Developmental Dynamics, 203(3), 253–310.
Sökeland, G., & Schumacher, U. (2019). The functional role of integrins during intra-and extravasation within the metastatic cascade. Molecular Cancer, 18(1), 12.
Barthel, S. R., Gavino, J. D., Descheny, L., & Dimitroff, C. J. (2007). Targeting selectins and selectin ligands in inflammation and cancer. Expert Opinion on Therapeutic Targets, 11(11), 1473–1491.
Auguste, P., Fallavollita, L., Wang, N., Burnier, J., Bikfalvi, A., & Brodt, P. (2007). The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. The American Journal of Pathology, 170(5), 1781–1792.
Kansas, G. S. (1996). Selectins and their ligands: current concepts and controversies. Blood, 88(9), 3259–3287.
Burdick, M. M., Chu, J. T., Godar, S., & Sackstein, R. (2006). HCELL is the major E-and L-selectin ligand expressed on LS174T colon carcinoma cells. Journal of Biological Chemistry, 281(20), 13899–13905.
Dimitroff, C. J., Lechpammer, M., Long-Woodward, D., & Kutok, J. L. (2004). Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Research, 64(15), 5261–5269.
Läubli, H., & Borsig, L. (2019). Selectins promote tumor metastasis. Seminars in Cancer Biology. Vol. 20. No. 3. Academic Press, 2010. https://www.sciencedirect.com/science/article/pii/S1044579X1000026X?casa_token=9LeVS2NAVuwAAAAA:OU_wIL6dQfFpiKRSo3RoICKbBd_MevziJlhk42tzujCFJb-2I7yyr8KD7KGymOc_PSIQhBGvpg.
Dimitroff, C. J., Descheny, L., Trujillo, N., Kim, R., Nguyen, V., Huang, W., Pienta, K. J., Kutok, J. L., & Rubin, M. A. (2005). Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Research, 65(13), 5750–5760.
Shenoy, A. K., & Lu, J. (2016). Cancer cells remodel themselves and vasculature to overcome the endothelial barrier. Cancer Letters, 380(2), 534–544.
Li, G., Satyamoorthy, K., & Herlyn, M. (2001). N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Research, 61(9), 3819–3825.
Garofalo, A., Chirivi, R. G., Foglieni, C., Pigott, R., Mortarini, R., Martin-Padura, I., Anichini, A., Gearing, A. J., Sanchez-Madrid, F., & Dejana, E. (1995). Involvement of the very late antigen 4 integrin on melanoma in interleukin 1-augmented experimental metastases. Cancer Research, 55(2), 414–419.
Kiefel, H., Bondong, S., Hazin, J., Ridinger, J., Schirmer, U., Riedle, S., & Altevogt, P. (2012). L1CAM: a major driver for tumor cell invasion and motility. Cell Adhesion & Migration, 6(4), 374–384.
Strell, C., Lang, K., Niggemann, B., Zaenker, K., & Entschladen, F. (2007). Surface molecules regulating rolling and adhesion to endothelium of neutrophil granulocytes and MDA-MB-468 breast carcinoma cells and their interaction. Cellular and Molecular Life Sciences, 64(24), 3306–3316.
Desgrosellier, J. S., & Cheresh, D. A. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews Cancer, 10(1), 9–22.
Chen, M. B., Lamar, J. M., Li, R., Hynes, R. O., & Kamm, R. D. (2016). Elucidation of the roles of tumor integrin β1 in the extravasation stage of the metastasis cascade. Cancer Research, 76(9), 2513–2524.
Li, F., Redick, S. D., Erickson, H. P., & Moy, V. T. (2003). Force measurements of the α5β1 integrin–fibronectin interaction. Biophysical Journal, 84(2), 1252–1262.
Kai, F., Drain, A. P., & Weaver, V. M. (2019). The extracellular matrix modulates the metastatic journey. Developmental Cell, 49(3), 332–346.
CHEN, W. T., & WANG, J. Y. (1999). Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Annals of the New York Academy of Sciences, 878(1), 361–371.
Paz, H., Pathak, N., & Yang, J. (2014). Invading one step at a time: the role of invadopodia in tumor metastasis. Oncogene, 33(33), 4193–4202.
Xu, R., Rai, A., Chen, M., Suwakulsiri, W., Greening, D. W., & Simpson, R. J. (2018). Extracellular vesicles in cancer—implications for future improvements in cancer care. Nature Reviews Clinical Oncology, 15(10), 617–638.
De Palma, M., Biziato, D., & Petrova, T. V. (2017). Microenvironmental regulation of tumour angiogenesis. Nature Reviews Cancer, 17(8), 457–474.
García-Román, J., & Zentella-Dehesa, A. (2013). Vascular permeability changes involved in tumor metastasis. Cancer Letters, 335(2), 259–269.
Jain, R. K., Martin, J. D., & Stylianopoulos, T. (2014). The role of mechanical forces in tumor growth and therapy. Annual Review of Biomedical Engineering, 16, 321–346.
Stroka, K. M., & Aranda-Espinoza, H. (2010). A biophysical view of the interplay between mechanical forces and signaling pathways during transendothelial cell migration. The FEBS Journal, 277(5), 1145–1158.
Weaver, A. M. (2006). Invadopodia: Specialized cell structures for cancer invasion. Clinical & Experimental Metastasis, 23(2), 97–105.
Yamaguchi, H., & Condeelis, J. (2007). Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1773(5), 642–652.
Jacob, A., & Prekeris, R. (2015). The regulation of MMP targeting to invadopodia during cancer metastasis. Frontiers in Cell and Developmental Biology, 3, 4.
Stoletov, K., Montel, V., Lester, R. D., Gonias, S. L., & Klemke, R. (2007). High-resolution imaging of the dynamic tumor cell–vascular interface in transparent zebrafish. Proceedings of the National Academy of Sciences, 104(44), 17406–17411.
Wang, S., Li, E., Gao, Y., Wang, Y., Guo, Z., He, J., et al. (2013). Study on invadopodia formation for lung carcinoma invasion with a microfluidic 3D culture device. PLoS One, 8(2), e56448.
Liu, T., Li, C., Li, H., Zeng, S., Qin, J., & Lin, B. (2009). A microfluidic device for characterizing the invasion of cancer cells in 3-D matrix. Electrophoresis, 30(24), 4285–4291.
Eckert, M. A., & Yang, J. (2011). Targeting invadopodia to block breast cancer metastasis. Oncotarget, 2(7), 562–568.
Kaczmarek, A., Vandenabeele, P., & Krysko, D. V. (2013). Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity, 38(2), 209–223.
Kim, H., Chung, H., Kim, J., Choi, D. H., Shin, Y., Kang, Y. G., et al. (2019). Macrophages-triggered sequential remodeling of endothelium-interstitial matrix to form pre-metastatic niche in microfluidic tumor microenvironment. Advanced Science, 6(11), 1900195.
Lee, T.-H., Avraham, H. K., Jiang, S., & Avraham, S. (2003). Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. Journal of Biological Chemistry, 278(7), 5277–5284.
Weis, S., Cui, J., Barnes, L., & Cheresh, D. (2004). Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. The Journal of Cell Biology, 167(2), 223–229.
Anderberg, C., Cunha, S. I., Zhai, Z., Cortez, E., Pardali, E., Johnson, J. R., Franco, M., Páez-Ribes, M., Cordiner, R., Fuxe, J., Johansson, B. R., Goumans, M. J., Casanovas, O., ten Dijke, P., Arthur, H. M., & Pietras, K. (2013). Deficiency for endoglin in tumor vasculature weakens the endothelial barrier to metastatic dissemination. Journal of Experimental Medicine, 210(3), 563–579.
Roussos, E. T., Condeelis, J. S., & Patsialou, A. (2011). Chemotaxis in cancer. Nature Reviews Cancer, 11(8), 573–587.
Padua, D., Zhang, X. H.-F., Wang, Q., Nadal, C., Gerald, W. L., Gomis, R. R., & Massagué, J. (2008). TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell, 133(1), 66–77.
Wolf, M. J., Hoos, A., Bauer, J., Boettcher, S., Knust, M., Weber, A., Simonavicius, N., Schneider, C., Lang, M., Stürzl, M., Croner, R. S., Konrad, A., Manz, M. G., Moch, H., Aguzzi, A., van Loo, G., Pasparakis, M., Prinz, M., Borsig, L., & Heikenwalder, M. (2012). Endothelial CCR2 signaling induced by colon carcinoma cells enables extravasation via the JAK2-Stat5 and p38MAPK pathway. Cancer Cell, 22(1), 91–105.
Wendel, C., Hemping-Bovenkerk, A., Krasnyanska, J., Mees, S. T., Kochetkova, M., Stoeppeler, S., & Haier, J. (2012). CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS One, 7(1), e30046.
Karki, P., & Birukov, K. G. (2018). Lipid mediators in the regulation of endothelial barriers. Tissue Barriers, 6(1), e1385573.
Freyssinet, J.-M., & Toti, F. (2010). Formation of procoagulant microparticles and properties. Thrombosis Research, 125, S46–S48.
Gay, L. J., & Felding-Habermann, B. (2011). Contribution of platelets to tumour metastasis. Nature Reviews Cancer, 11(2), 123–134.
Takuwa, Y. (2002). Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1582(1–3), 112–120.
McVerry, B. J., & Garcia, J. G. (2005). In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cellular Signalling, 17(2), 131–139.
Yin, F., & Watsky, M. A. (2005). LPA and S1P increase corneal epithelial and endothelial cell transcellular resistance. Investigative Ophthalmology & Visual Science, 46(6), 1927–1933.
Sarker, M. H., Hu, D. E., & Fraser, P. A. (2010). Regulation of cerebromicrovascular permeability by lysophosphatidic acid. Microcirculation, 17(1), 39–46.
Zhou, W., Fong, M. Y., Min, Y., Somlo, G., Liu, L., Palomares, M. R., Yu, Y., Chow, A., O’Connor, S. T. F., Chin, A. R., Yen, Y., Wang, Y., Marcusson, E. G., Chu, P., Wu, J., Wu, X., Li, A. X., Li, Z., Gao, H., Ren, X., Boldin, M. P., Lin, P. C., & Wang, S. E. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 25(4), 501–515.
Lin, Y., Zhang, C., Xiang, P., Shen, J., Sun, W., & Yu, H. (2020). Exosomes derived from HeLa cells break down vascular integrity by triggering endoplasmic reticulum stress in endothelial cells. Journal of Extracellular Vesicles, 9(1), 1722385.
Kikuchi, S., Yoshioka, Y., Prieto-Vila, M., & Ochiya, T. (2019). Involvement of extracellular vesicles in vascular-related functions in cancer progression and metastasis. International Journal of Molecular Sciences, 20(10), 2584.
Zeng, Z., Li, Y., Pan, Y., Lan, X., Song, F., Sun, J., et al. (2018). Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nature Communications, 9(1), 1–14.
Hsu, Y., Hung, J., Chang, W., Lin, Y., Pan, Y., Tsai, P., et al. (2017). Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene, 36(34), 4929–4942.
Wirtz, D., Konstantopoulos, K., & Searson, P. C. (2011). The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nature Reviews Cancer, 11(7), 512–522.
Thomas, S. N., Zhu, F., Schnaar, R. L., Alves, C. S., & Konstantopoulos, K. (2008). Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E-and L-selectin in shear flow. Journal of Biological Chemistry, 283(23), 15647–15655.
Wojciak-Stothard, B., & Ridley, A. J. (2003). Shear stress–induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. The Journal of Cell Biology, 161(2), 429–439.
Lapis, K., Paku, S., & Liotta, L. (1988). Endothelialization of embolized tumor cells during metastasis formation. Clinical & Experimental Metastasis, 6(1), 73–89.
Paku, S., Döme, B., Tóth, R., & Timár, J. (2000). Organ-specificity of the extravasation process: an ultrastructural study. Clinical & Experimental Metastasis, 18(6), 481–492.
Krüger-Genge, A., Blocki, A., Franke, R.-P., & Jung, F. (2019). Vascular endothelial cell biology: an update. International Journal of Molecular Sciences, 20(18), 4411.
Félétou, M. (2011) The endothelium, part I: multiple functions of the endothelial cells--focus on endothelium-derived vasoactive mediators. In Colloquium series on integrated systems physiology: From molecule to function (vol. 3–4, pp. 1–306). San Rafael: Morgan & Claypool Life Sciences.
Stamatovic, S. M., Keep, R. F., & Andjelkovic, A. V. (2008). Brain endothelial cell-cell junctions: how to “open” the blood brain barrier. Current Neuropharmacology, 6(3), 179–192.
Lam, C. K., Yoo, T., Hiner, B., Liu, Z., & Grutzendler, J. (2010). Embolus extravasation is an alternative mechanism for cerebral microvascular recanalization. Nature, 465(7297), 478–482.
Grutzendler, J., Murikinati, S., Hiner, B., Ji, L., Lam, C. K., Yoo, T., et al. (2014). Angiophagy prevents early embolus washout but recanalizes microvessels through embolus extravasation. Science Translational Medicine, 6(226), 226ra231.
Zhou, T., Zheng, Y., Sun, L., Badea, S. R., Jin, Y., Liu, Y., Rolfe, A. J., Sun, H., Wang, X., Cheng, Z., Huang, Z., Zhao, N., Sun, X., Li, J., Fan, J., Lee, C., Megraw, T. L., Wu, W., Wang, G., & Ren, Y. (2019). Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nature Neuroscience, 22(3), 421–435.
Dini, L., Lentini, A., Diez, G. D., Rocha, M., Falasca, L., Serafino, L., et al. (1995). Phagocytosis of apoptotic bodies by liver endothelial cells. Journal of Cell Science, 108(3), 967–973.
Steffan, A. M., Gendrault, J. L., McCuskey, R. S., McCuskey, P. A., & Kirn, A. (1986). Phagocytosis, an unrecognized property of murine endothelial liver cells. Hepatology, 6(5), 830–836.
Rengarajan, M., Hayer, A., & Theriot, J. A. (2016). Endothelial cells use a formin-dependent phagocytosis-like process to internalize the bacterium Listeria monocytogenes. PLoS Pathogens, 12(5), e1005603.
Maniotis, A. J., Folberg, R., Hess, A., Seftor, E. A., Gardner, L. M., Pe’er, J., et al. (1999). Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. The American Journal of Pathology, 155(3), 739–752.
Hendrix, M. J., Seftor, E. A., Hess, A. R., & Seftor, R. E. (2003). Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nature Reviews Cancer, 3(6), 411–421.
Cima, I., Kong, S. L., Sengupta, D., Tan, I. B., Phyo, W. M., Lee, D., et al. (2016). Tumor-derived circulating endothelial cell clusters in colorectal cancer. Science Translational Medicine, 8(345), 345ra389.
Esposito, M., Magnani, J. L., & Kang, Y. (2014). Exploration of a potent E-selectin antagonist (GMI-1271) as a potential novel therapeutic for treating breast cancer metastasis to the bone and lung. AACR. https://cancerres.aacrjournals.org/content/74/19_Supplement/4039.short.
Steele, M. M., Radhakrishnan, P., Magnani, J. L., & Hollingsworth, M. A. (2014). A small molecule glycomimetic antagonist of E-selectin (GMI-1271) prevents pancreatic tumor metastasis and offers a novel treatment for improved efficacy of chemotherapy. pp. 4503–4503. https://cancerres.aacrjournals.org/content/74/19_Supplement/4503.short.
Bendas, G., & Borsig, L. (2012). Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins. International Journal of Cell Biology, 2012, 676731. https://doi.org/10.1155/2012/676
Hostettler, N., Naggi, A., Torri, G., Ishai-Michaeli, R., Casu, B., Vlodavsky, I., & Borsig, L. (2007). P-selectin-and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. The FASEB Journal, 21(13), 3562–3572.
Fritzsche, J., Simonis, D., & Bendas, G. (2008). Melanoma cell adhesion can be blocked by heparin in vitro: suggestion of VLA-4 as a novel target for antimetastatic approaches. Thrombosis and Haemostasis, 100(12), 1166–1175.
Zhang, C., Liu, Y., Gao, Y., Shen, J., Zheng, S., Wei, M., & Zeng, X. L. (2009). Modified heparins inhibit integrin αIIbβ3 mediated adhesion of melanoma cells to platelets in vitro and in vivo. International Journal of Cancer, 125(9), 2058–2065.
Kakkar, A., & Macbeth, F. (2010). Antithrombotic therapy and survival in patients with malignant disease. British Journal of Cancer, 102(1), S24–S29.
Huang, H., Bhat, A., Woodnutt, G., & Lappe, R. (2010). Targeting the ANGPT–TIE2 pathway in malignancy. Nature Reviews Cancer, 10(8), 575–585.
Biel, N. M., & Siemann, D. W. (2016). Targeting the Angiopoietin-2/Tie-2 axis in conjunction with VEGF signal interference. Cancer Letters, 380(2), 525–533.
Wu, F. T., Lee, C. R., Bogdanovic, E., Prodeus, A., Gariépy, J., & Kerbel, R. S. (2015). Vasculotide reduces endothelial permeability and tumor cell extravasation in the absence of binding to or agonistic activation of Tie2. EMBO Molecular Medicine, 7(6), 770–787.
Zheng, N., Chen, J., Liu, W., Liu, J., Li, T., Chen, H., Wang, J., & Jia, L. (2017). Mifepristone inhibits ovarian cancer metastasis by intervening in SDF-1/CXCR4 chemokine axis. Oncotarget, 8(35), 59123–59135.
Uchida, D., Kuribayashi, N., Kinouchi, M., Sawatani, Y., Shimura, M., Mori, T., et al. (2018). Effect of a novel orally bioavailable CXCR4 inhibitor, AMD070, on the metastasis of oral cancer cells. Oncology Reports, 40(1), 303–308.
Schlesinger, M. (2018). Role of platelets and platelet receptors in cancer metastasis. Journal of Hematology & Oncology, 11(1), 1–15.
Elaskalani, O., Berndt, M. C., Falasca, M., & Metharom, P. (2017). Targeting platelets for the treatment of cancer. Cancers, 9(7), 94.
Rothwell, P. M., Wilson, M., Price, J. F., Belch, J. F., Meade, T. W., & Mehta, Z. (2012). Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. The Lancet, 379(9826), 1591–1601.
Lieu, C. H., Tan, A.-C., Leong, S., Diamond, J. R., & Eckhardt, S. G. (2013). From bench to bedside: lessons learned in translating preclinical studies in cancer drug development. Journal of the National Cancer Institute, 105(19), 1441–1456.
Gould, S. E., Junttila, M. R., & de Sauvage, F. J. (2015). Translational value of mouse models in oncology drug development. Nature Medicine, 21(5), 431–439.
Ozsvári, B., Lamb, R., & Lisanti, M. P. (2016). Repurposing of FDA-approved drugs against cancer–focus on metastasis. Aging (Albany NY), 8(4), 567–568.
Klangjorhor, J., Chaiyawat, P., Teeyakasem, P., Sirikaew, N., Phanphaisarn, A., Settakorn, J., Lirdprapamongkol, K., Yama, S., Svasti, J., & Pruksakorn, D. (2020). Mycophenolic acid is a drug with the potential to be repurposed for suppressing tumor growth and metastasis in osteosarcoma treatment. International Journal of Cancer, 146(12), 3397–3409.
Hachey, S. J., & Hughes, C. C. (2018). Applications of tumor chip technology. Lab on a Chip, 18(19), 2893–2912.
Acknowledgements
This work was supported by grants from the National Institutes of Health (HL137093).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, X., Cheng, K. Visualizing cancer extravasation: from mechanistic studies to drug development. Cancer Metastasis Rev 40, 71–88 (2021). https://doi.org/10.1007/s10555-020-09942-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09942-2