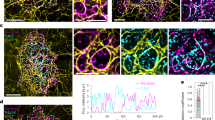
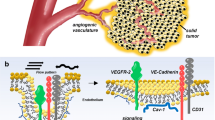
Abstract
Tumor stiffening is a hallmark of malignancy that actively drives tumor progression and aggressiveness. Recent research has shed light onto several molecular underpinnings of this biomechanical process, which has a reciprocal crosstalk between tumor cells, stromal fibroblasts, and extracellular matrix remodeling at its core. This dynamic communication shapes the tumor microenvironment; significantly determines disease features including therapeutic resistance, relapse, or metastasis; and potentially holds the key for novel antitumor strategies. Caveolae and their components emerge as integrators of different aspects of cell function, mechanotransduction, and ECM–cell interaction. Here, we review our current knowledge on the several pivotal roles of the essential caveolar component caveolin-1 in this multidirectional biomechanical crosstalk and highlight standing questions in the field.


Similar content being viewed by others
References
Young, J. S., Llumsden, C. E., & Stalker, A. L. (1950). The significance of the “tissue pressure” of normal testicular and of neoplastic (Brown-Pearce carcinoma) tissue in the rabbit. The Journal of Pathology and Bacteriology, 62(3), 313–333. https://doi.org/10.1002/path.1700620303.
Northcott, J. M., Dean, I. S., Mouw, J. K., & Weaver, V. M. (2018). Feeling stress: the mechanics of cancer progression and aggression. Frontiers in Cell and Developmental Biology. https://doi.org/10.3389/fcell.2018.00017.
Kai, F. B., Laklai, H., & Weaver, V. M. (2016). Force matters: biomechanical regulation of cell invasion and migration in disease. Trends in Cell Biology, 26(7), 486–497. https://doi.org/10.1016/j.tcb.2016.03.007.
Paszek, M. J., Zahir, N., Johnson, K. R., Lakins, J. N., Rozenberg, G. I., Gefen, A., et al. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell, 8(3), 241–254. https://doi.org/10.1016/j.ccr.2005.08.010.
Lin, X., Shi, Y., Cao, Y., & Liu, W. (2016). Recent progress in stem cell differentiation directed by material and mechanical cues. Biomedical materials (Bristol, England), 11(1), 14109. https://doi.org/10.1088/1748-6041/11/1/014109.
Paszek, M. J., DuFort, C. C., Rossier, O., Bainer, R., Mouw, J. K., Godula, K., et al. (2014). The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature, 511(7509), 319–325. https://doi.org/10.1038/nature13535.
Anastasiou, O., Hadjisavva, R., & Skourides, P. A. (2020). Mitotic cell responses to substrate topological cues are independent of the molecular nature of adhesion. Science Signaling, 13(620), eaax9940. https://doi.org/10.1126/scisignal.aax9940.
Joyce, M. H., Lu, C., James, E. R., Hegab, R., Allen, S. C., Suggs, L. J., & Brock, A. (2018). Phenotypic basis for matrix stiffness-dependent chemoresistance of breast cancer cells to doxorubicin. Frontiers in Oncology, 8, 337. https://doi.org/10.3389/fonc.2018.00337.
Sewell-Loftin, M. K., Bayer, S. V. H., Crist, E., Hughes, T., Joison, S. M., Longmore, G. D., & George, S. C. (2017). Cancer-associated fibroblasts support vascular growth through mechanical force. Scientific Reports, 7(1), 12574. https://doi.org/10.1038/s41598-017-13006-x.
Reid, S. E., Kay, E. J., Neilson, L. J., Henze, A.-T., Serneels, J., McGhee, E. J., et al. (2017). Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. The EMBO Journal, 36(16), 2373–2389. https://doi.org/10.15252/embj.201694912.
Provenzano, P. P., Inman, D. R., Eliceiri, K. W., Trier, S. M., & Keely, P. J. (2008). Contact guidance mediated three-dimensional cell migration is regulated by rho/ROCK-dependent matrix reorganization. Biophysical Journal, 95(11), 5374–5384. https://doi.org/10.1529/biophysj.108.133116.
Liu, J., Tan, Y., Zhang, H., Zhang, Y., Xu, P., Chen, J., et al. (2012). Soft fibrin gels promote selection and growth of tumorigenic cells. Nature Materials, 11(8), 734–741. https://doi.org/10.1038/nmat3361.
Bordeleau, F., Mason, B. N., Lollis, E. M., Mazzola, M., Zanotelli, M. R., Somasegar, S., et al. (2017). Matrix stiffening promotes a tumor vasculature phenotype. Proceedings of the National Academy of Sciences of the United States of America, 114(3), 492–497. https://doi.org/10.1073/pnas.1613855114.
Lampi, M. C., & Reinhart-king, C. A. (2018). Targeting extracellular matrix stiffness to attenuate disease: from molecular mechanisms to clinical trials. Science Translational Medicine, 0475(January), 1–15.
Nevala-Plagemann, C., Hidalgo, M., & Garrido-Laguna, I. (2020). From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nature Reviews. Clinical Oncology, 17(2), 108–123. https://doi.org/10.1038/s41571-019-0281-6.
Mouw, J. K., Ou, G., & Weaver, V. M. (2014). Extracellular matrix assembly: a multiscale deconstruction. Nature Reviews. Molecular Cell Biology, 15(12), 771–785. https://doi.org/10.1038/nrm3902.
Malik, R., Lelkes, P. I., & Cukierman, E. (2015). Biomechanical and biochemical remodeling of stromal extracellular matrix in cancer. Trends in Biotechnology, 33(4), 230–236. https://doi.org/10.1016/j.tibtech.2015.01.004.
Gopal, S., Veracini, L., Grall, D., Butori, C., Schaub, S., Audebert, S., et al. (2017). Fibronectin-guided migration of carcinoma collectives. Nature Communications, 8(1), 14105. https://doi.org/10.1038/ncomms14105.
Leppänen, J., Lindholm, V., Isohookana, J., Haapasaari, K.-M., Karihtala, P., Lehenkari, P. P., et al. (2019). Tenascin C, fibronectin, and tumor-stroma ratio in pancreatic ductal adenocarcinoma. Pancreas, 48(1) Retrieved from https://journals.lww.com/pancreasjournal/Fulltext/2019/01000/Tenascin_C,_Fibronectin,_and_Tumor_Stroma_Ratio_in.5.aspx.
Wartiovaara, J., Leivo, I., Virtanen, I., Vaheri, A., & Graham, C. F. (1978). Appearance of fibronectin during differentiation of mouse teratocarcinoma in vitro. Nature, 272(5651), 355–356. https://doi.org/10.1038/272355a0.
Chambers, A. F., Behrend, E. I., Wilson, S. M., & Denhardt, D. T. (1992). Induction of expression of osteopontin (OPN; secreted phosphoprotein) in metastatic, ras-transformed NIH 3T3 cells. Anticancer Research, 12(1), 43–47.
Hebert, J. D., Myers, S. A., Naba, A., Abbruzzese, G., Lamar, J. M., Carr, S. A., & Hynes, R. O. (2020). Proteomic profiling of the ECM of xenograft breast cancer metastases in different organs reveals distinct metastatic niches. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-19-2961.
Naba, A., Clauser, K. R., Ding, H., Whittaker, C. A., Carr, S. A., & Hynes, R. O. (2016). The extracellular matrix: tools and insights for the “omics” era. Matrix Biology: Journal of the International Society for Matrix Biology, 49, 10–24. https://doi.org/10.1016/j.matbio.2015.06.003.
Oudin, M. J., Jonas, O., Kosciuk, T., Broye, L. C., Guido, B. C., Wyckoff, J., et al. (2016). Tumor cell-driven extracellular matrix remodeling drives haptotaxis during metastatic progression. Cancer Discovery, 6(5), 516–531. https://doi.org/10.1158/2159-8290.CD-15-1183.
Tian, C., Clauser, K. R., Ohlund, D., Rickelt, S., Huang, Y., Gupta, M., et al. (2019). Proteomic analyses of ECM during pancreatic ductal adenocarcinoma progression reveal different contributions by tumor and stromal cells. Proceedings of the National Academy of Sciences of the United States of America, 116(39), 19609–19618. https://doi.org/10.1073/pnas.1908626116.
Tian, C., Ohlund, D., Rickelt, S., Lidstrom, T., Huang, Y., Hao, L., et al. (2020). Cancer cell-derived matrisome proteins promote metastasis in pancreatic ductal adenocarcinoma. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-19-2578.
Gocheva, V., Naba, A., Bhutkar, A., Guardia, T., Miller, K. M., Li, C. M.-C., et al. (2017). Quantitative proteomics identify Tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proceedings of the National Academy of Sciences of the United States of America, 114(28), E5625–E5634. https://doi.org/10.1073/pnas.1707054114.
Kaur, A., Ecker, B. L., Douglass, S. M., Kugel 3rd, C. H., Webster, M. R., Almeida, F. V., et al. (2019). Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discovery, 9(1), 64–81. https://doi.org/10.1158/2159-8290.CD-18-0193.
Sun, Z., Schwenzer, A., Rupp, T., Murdamoothoo, D., Vegliante, R., Lefebvre, O., et al. (2018). Tenascin-C promotes tumor cell migration and metastasis through integrin α9β1-mediated YAP inhibition. Cancer Research, 78(4), 950–961. https://doi.org/10.1158/0008-5472.CAN-17-1597.
Cai, J., Lu, W., Du, S., Guo, Z., Wang, H., Wei, W., & Shen, X. (2018). Tenascin-C modulates cell cycle progression to enhance tumour cell proliferation through AKT/FOXO1 signalling in pancreatic cancer. Journal of Cancer, 9(23), 4449–4462. https://doi.org/10.7150/jca.25926.
Castello, L. M., Raineri, D., Salmi, L., Clemente, N., Vaschetto, R., Quaglia, M., et al. (2017). Osteopontin at the crossroads of inflammation and tumor progression. Mediators of Inflammation, 2017, 4049098. https://doi.org/10.1155/2017/4049098.
Van Obberghen-Schilling, E., Tucker, R. P., Saupe, F., Gasser, I., Cseh, B., & Orend, G. (2011). Fibronectin and tenascin-C: accomplices in vascular morphogenesis during development and tumor growth. The International Journal of Developmental Biology, 55(4–5), 511–525. https://doi.org/10.1387/ijdb.103243eo.
Chang, J., & Chaudhuri, O. (2019). Beyond proteases: basement membrane mechanics and cancer invasion. The Journal of Cell Biology, 218(8), 2456–2469. https://doi.org/10.1083/jcb.201903066.
Wen, Q., & Janmey, P. A. (2013). Effects of non-linearity on cell-ECM interactions. Experimental Cell Research, 319(16), 2481–2489. https://doi.org/10.1016/j.yexcr.2013.05.017.
Conklin, M. W., Eickhoff, J. C., Riching, K. M., Pehlke, C. A., Eliceiri, K. W., Provenzano, P. P., et al. (2011). Aligned collagen is a prognostic signature for survival in human breast carcinoma. The American Journal of Pathology, 178(3), 1221–1232. https://doi.org/10.1016/j.ajpath.2010.11.076.
Bredfeldt, J. S., Liu, Y., Pehlke, C. A., Conklin, M. W., Szulczewski, J. M., Inman, D. R., et al. (2014). Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. Journal of Biomedical Optics, 19(1), 16007. https://doi.org/10.1117/1.JBO.19.1.016007.
Mosher, D. F. (1993). Assembly of fibronectin into extracellular matrix. Current Opinion in Structural Biology, 3(2), 214–222. https://doi.org/10.1016/S0959-440X(05)80155-1.
Bonnans, C., Chou, J., & Werb, Z. (2014). Remodelling the extracellular matrix in development and disease. Nature Reviews. Molecular cell biology, 15(12), 786–801. https://doi.org/10.1038/nrm3904.
Wang, K., Wu, F., Seo, B. R., Fischbach, C., Chen, W., Hsu, L., & Gourdon, D. (2017). Breast cancer cells alter the dynamics of stromal fibronectin-collagen interactions. Matrix Biology, 60–61, 86–95. https://doi.org/10.1016/j.matbio.2016.08.001.
Erdogan, B., Ao, M., White, L. M., Means, A. L., Brewer, B. M., Yang, L., et al. (2017). Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. Journal of Cell Biology, 216(11), 3799–3816. https://doi.org/10.1083/jcb.201704053.
Cox, T. R., Bird, D., Baker, A.-M., Barker, H. E., Ho, M. W.-Y., Lang, G., & Erler, J. T. (2013). LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Research, 73(6), 1721–1732. https://doi.org/10.1158/0008-5472.CAN-12-2233.
Harburger, D. S., & Calderwood, D. A. (2009). Integrin signalling at a glance. Journal of Cell Science, 122(Pt 2), 159–163. https://doi.org/10.1242/jcs.018093.
Attieh, Y., Clark, A. G., Grass, C., Richon, S., Pocard, M., Mariani, P., et al. (2017). Cancer-associated fibroblasts lead tumor invasion through integrin-beta3-dependent fibronectin assembly. The Journal of Cell Biology, 216(11), 3509–3520. https://doi.org/10.1083/jcb.201702033.
Farrugia, A. J., & Calvo, F. (2017). Cdc42 regulates Cdc42EP3 function in cancer-associated fibroblasts. Small GTPases, 8(1), 49–57. https://doi.org/10.1080/21541248.2016.1194952.
Sahai, E., Astsaturov, I., Cukierman, E., DeNardo, D. G., Egeblad, M., Evans, R. M., et al. (2020). A framework for advancing our understanding of cancer-associated fibroblasts. Nature Reviews. Cancer, 20(3), 174–186. https://doi.org/10.1038/s41568-019-0238-1.
Abercrombie, M., & Heaysman, J. E. M. (1953). Observations on the social behaviour of cells in tissue culture: I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Experimental Cell Research, 5(1), 111–131. https://doi.org/10.1016/0014-4827(53)90098-6.
Theveneau, E., Steventon, B., Scarpa, E., Garcia, S., Trepat, X., Streit, A., & Mayor, R. (2013). Chase-and-run between adjacent cell populations promotes directional collective migration. Nature Cell Biology, 15(7), 763–772. https://doi.org/10.1038/ncb2772.
Elsdale, T. R. (1968). Parallel orientation of fibroblasts in vitro. Experimental Cell Research, 51(2), 439–450. https://doi.org/10.1016/0014-4827(68)90134-1.
Davis, J. R., Luchici, A., Mosis, F., Thackery, J., Salazar, J. A., Mao, Y., et al. (2015). Inter-cellular forces orchestrate contact inhibition of locomotion. Cell, 161(2), 361–373. https://doi.org/10.1016/j.cell.2015.02.015.
Park, D., Wershof, E., Boeing, S., Labernadie, A., Jenkins, R. P., George, S., et al. (2020). Extracellular matrix anisotropy is determined by TFAP2C-dependent regulation of cell collisions. Nature Materials, 19(2), 227–238. https://doi.org/10.1038/s41563-019-0504-3.
Bissell, M. J., Hall, H. G., & Parry, G. (1982). How does the extracellular matrix direct gene expression? Journal of Theoretical Biology, 99(1), 31–68. https://doi.org/10.1016/0022-5193(82)90388-5.
Nijhout, H. F., Best, J. A., & Reed, M. C. (2019). Systems biology of robustness and homeostatic mechanisms. Wiley Interdisciplinary Reviews. Systems Biology and Medicine, 11(3), e1440. https://doi.org/10.1002/wsbm.1440.
Xu, R., Boudreau, A., & Bissell, M. J. (2009). Tissue architecture and function: dynamic reciprocity via extra- and intra-cellular matrices. Cancer and Metastasis Reviews, 28(1), 167–176. https://doi.org/10.1007/s10555-008-9178-z.
Nelson, C. M., & Bissell, M. J. (2006). Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annual Review of Cell and Developmental Biology, 22(1), 287–309. https://doi.org/10.1146/annurev.cellbio.22.010305.104315.
LeBleu, V. S., & Neilson, E. G. (2020). Origin and functional heterogeneity of fibroblasts. FASEB Journal, (January), 1–18. https://doi.org/10.1096/fj.201903188R.
Öhlund, D., Elyada, E., & Tuveson, D. (2014). Fibroblast heterogeneity in the cancer wound. Journal of Experimental Medicine, 211(8), 1503–1523. https://doi.org/10.1084/jem.20140692.
Micallef, L., Vedrenne, N., Billet, F., Coulomb, B., Darby, I. A., & Desmoulière, A. (2012). The myofibroblast, multiple origins for major roles in normal and pathological tissue repair. Fibrogenesis & Tissue Repair, 5(Suppl 1), S5–S5. https://doi.org/10.1186/1755-1536-5-S1-S5.
Cox, T. R., & Erler, J. T. (2011). Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Disease Models & Mechanisms, 4(2), 165–178. https://doi.org/10.1242/dmm.004077.
Kuzet, S. E., & Gaggioli, C. (2016). Fibroblast activation in cancer: when seed fertilizes soil. Cell and Tissue Research, 365(3), 607–619. https://doi.org/10.1007/s00441-016-2467-x.
Rettig, W. J., Garin-Chesa, P., Healey, J. H., Su, S. L., Ozer, H. L., Schwab, M., et al. (1993). Regulation and heteromeric structure of the fibroblast activation protein in normal and transformed cells of mesenchymal and neuroectodermal origin. Cancer Research, 53(14), 3327–3335.
Tomasek, J. J., Gabbiani, G., Hinz, B., Chaponnier, C., & Brown, R. A. (2002). Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature Reviews. Molecular Cell Biology, 3(5), 349–363. https://doi.org/10.1038/nrm809.
Hu, M., Yao, J., Cai, L., Bachman, K. E., van den Brûle, F., Velculescu, V., & Polyak, K. (2005). Distinct epigenetic changes in the stromal cells of breast cancers. Nature Genetics, 37(8), 899–905. https://doi.org/10.1038/ng1596.
Vizoso, M., Puig, M., Carmona, F. J., Maqueda, M., Velasquez, A., Gomez, A., et al. (2015). Aberrant DNA methylation in non-small cell lung cancer-associated fibroblasts. Carcinogenesis, 36(12), 1453–1463. https://doi.org/10.1093/carcin/bgv146.
Webber, J. P., Spary, L. K., Sanders, A. J., Chowdhury, R., Jiang, W. G., Steadman, R., et al. (2015). Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene, 34(3), 290–302. https://doi.org/10.1038/onc.2013.560.
Amatangelo, M. D., Bassi, D. E., Klein-Szanto, A. J. P., & Cukierman, E. (2005). Stroma-derived three-dimensional matrices are necessary and sufficient to promote desmoplastic differentiation of normal fibroblasts. The American Journal of Pathology, 167(2), 475–488. https://doi.org/10.1016/S0002-9440(10)62991-4.
Calvo, F., Ege, N., Grande-Garcia, A., Hooper, S., Jenkins, R. P., Chaudhry, S. I., et al. (2013). Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nature Cell Biology, 15(6), 637–646. https://doi.org/10.1038/ncb2756.
Zhao, B., Wei, X., Li, W., Udan, R. S., Yang, Q., Kim, J., et al. (2007). Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & Development, 21(21), 2747–2761. https://doi.org/10.1101/gad.1602907.
Yu, F.-X., Zhao, B., & Guan, K.-L. (2015). Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell, 163(4), 811–828. https://doi.org/10.1016/j.cell.2015.10.044.
Piccolo, S., Dupont, S., & Cordenonsi, M. (2014). The biology of YAP/TAZ: Hippo signaling and beyond. Physiological Reviews, 94(4), 1287–1312. https://doi.org/10.1152/physrev.00005.2014.
Sorrentino, G., Ruggeri, N., Specchia, V., Cordenonsi, M., Mano, M., Dupont, S., et al. (2014). Metabolic control of YAP and TAZ by the mevalonate pathway. Nature Cell Biology, 16(4), 357–366. https://doi.org/10.1038/ncb2936.
Koo, J. H., & Guan, K.-L. (2018). Interplay between YAP/TAZ and metabolism. Cell Metabolism, 28(2), 196–206. https://doi.org/10.1016/j.cmet.2018.07.010.
Romani, P., Brian, I., Santinon, G., Pocaterra, A., Audano, M., Pedretti, S., et al. (2019). Extracellular matrix mechanical cues regulate lipid metabolism through Lipin-1 and SREBP. Nature Cell Biology, 21(3), 338–347. https://doi.org/10.1038/s41556-018-0270-5.
Zhong, W., Tian, K., Zheng, X., Li, L., Zhang, W., Wang, S., & Qin, J. (2013). Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by yes-associated protein. Stem Cells and Development, 22(14), 2083–2093. https://doi.org/10.1089/scd.2012.0685.
Dupont, S., Morsut, L., Aragona, M., Enzo, E., Giulitti, S., Cordenonsi, M., et al. (2011). Role of YAP/TAZ in mechanotransduction. Nature, 474(7350), 179–183. https://doi.org/10.1038/nature10137.
Codelia, V. A., Sun, G., & Irvine, K. D. (2014). Regulation of YAP by mechanical strain through Jnk and hippo signaling. Current Biology, 24(17), 2012–2017. https://doi.org/10.1016/j.cub.2014.07.034.
Zhao, B., Ye, X., Yu, J., Li, L., Li, W., Li, S., et al. (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes & Development, 22(14), 1962–1971. https://doi.org/10.1101/gad.1664408.
Ferrari, N., Ranftl, R., Chicherova, I., Slaven, N. D., Moeendarbary, E., Farrugia, A. J., et al. (2019). Dickkopf-3 links HSF1 and YAP/TAZ signalling to control aggressive behaviours in cancer-associated fibroblasts. Nature Communications, 10(1), 130. https://doi.org/10.1038/s41467-018-07987-0.
Warburg, O. (1925). The metabolism of carcinoma cells 1. The Journal of Cancer Research. https://doi.org/10.1158/jcr.1925.148.
Warburg, O., Wind, F., & Negelein, E. (1926). Über den Stoffwechsel von Tumoren im Körper. Klinische Wochenschrift. https://doi.org/10.1007/BF01726240.
Waddington, C. H. (1935). Cancer and the theory of organisers. Nature. https://doi.org/10.1038/135606a0.
Nowell, P. C., & Hungerford, D. A. (1960). Chromosome studies on normal and leukemic human leukocytes. Journal of the National Cancer Institute. https://doi.org/10.1093/jnci/25.1.85.
Duesberg, P. H., & Vogt, P. K. (1970). Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.67.4.1673.
Bister, K. (2015). Discovery of oncogenes: the advent of molecular cancer research. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.1521145112.
Scolnick, E. M., Rands, E., Williams, D., & Parks, W. P. (1973). Studies on the nucleic acid sequences of Kirsten sarcoma virus: a model for formation of a mammalian RNA-containing sarcoma virus. Journal of Virology. https://doi.org/10.1128/jvi.12.3.458-463.1973.
Scolnick, E. M., & Parks, W. P. (1974). Harvey sarcoma virus: a second murine type C sarcoma virus with rat genetic information. Journal of Virology. https://doi.org/10.1128/jvi.13.6.1211-1219.1974.
Stehelin, D., Varmus, H. E., Bishop, J. M., & Vogt, P. K. (1976). DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. https://doi.org/10.1038/260170a0.
Duesberg, P. H., & Vogt, P. K. (1979). Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.76.4.1633.
Hu, S. S. F., Lai, M. M. C., & Vogt, P. K. (1979). Genome of avian myelocytomatosis virus MC29: analysis by heteroduplex mapping. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.76.3.1265.
Dolberg, D. S., & Bissell, M. J. (1984). Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. https://doi.org/10.1038/309552a0.
Varmus, H., & Weinberg, R. A. (1994). Genes and the biology of cancer. The Quarterly Review of Biology. https://doi.org/10.1086/418732.
Rhim, A. D., Oberstein, P. E., Thomas, D. H., Mirek, E. T., Palermo, C. F., Sastra, S. A., et al. (2014). Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell, 25(6), 735–747. https://doi.org/10.1016/j.ccr.2014.04.021.
Brunner, S. F., Roberts, N. D., Wylie, L. A., Moore, L., Aitken, S. J., Davies, S. E., et al. (2019). Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature, 574(7779), 538–542. https://doi.org/10.1038/s41586-019-1670-9.
Martincorena, I., Roshan, A., Gerstung, M., Ellis, P., Van Loo, P., McLaren, S., et al. (2015). Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science (New York, N.Y.), 348(6237), 880–886. https://doi.org/10.1126/science.aaa6806.
Weaver, V. M., Lelièvre, S., Lakins, J. N., Chrenek, M. A., Jones, J. C. R., Giancotti, F., et al. (2002). β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. https://doi.org/10.1016/S1535-6108(02)00125-3.
Weaver, V. M., Petersen, O. W., Wang, F., Larabell, C. A., Briand, P., Damsky, C., & Bissell, M. J. (1997). Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. Journal of Cell Biology. https://doi.org/10.1083/jcb.137.1.231.
Yu, H., Mouw, J. K., & Weaver, V. M. (2011). Forcing form and function: biomechanical regulation of tumor evolution. Trends in Cell Biology, 21(1), 47–56. https://doi.org/10.1016/j.tcb.2010.08.015.
Northey, J. J., Przybyla, L., & Weaver, V. M. (2017). Tissue force programs cell fate and tumor aggression. Cancer Discovery. https://doi.org/10.1158/2159-8290.CD-16-0733.
Leight, J. L., Wozniak, M. A., Chen, S., Lynch, M. L., & Chen, C. S. (2012). Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Molecular Biology of the Cell, 23(5), 781–791. https://doi.org/10.1091/mbc.E11-06-0537.
Butcher, D. T., Alliston, T., & Weaver, V. M. (2009). A tense situation: forcing tumour progression. Nature Reviews. Cancer, 9(2), 108–122. https://doi.org/10.1038/nrc2544.
Azevedo, A. S., Follain, G., Patthabhiraman, S., Harlepp, S., & Goetz, J. G. (2015). Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both? Cell Adhesion & Migration, 9(5), 345–356. https://doi.org/10.1080/19336918.2015.1059563.
Huang, Q., Hu, X., He, W., Zhao, Y., Hao, S., Wu, Q., et al. (2018). Fluid shear stress and tumor metastasis. American Journal of Cancer Research, 8(5), 763–777 Retrieved from https://pubmed.ncbi.nlm.nih.gov/29888101.
Uhler, C., & Shivashankar, G. V. (2017). Regulation of genome organization and gene expression by nuclear mechanotransduction. Nature Reviews. Molecular Cell Biology, 18(12), 717–727. https://doi.org/10.1038/nrm.2017.101.
Nishioka, N., Inoue, K., Adachi, K., Kiyonari, H., Ota, M., Ralston, A., et al. (2009). The hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Developmental Cell. https://doi.org/10.1016/j.devcel.2009.02.003.
Rosado-Olivieri, E. A., Anderson, K., Kenty, J. H., & Melton, D. A. (2019). YAP inhibition enhances the differentiation of functional stem cell-derived insulin-producing β cells. Nature Communications. https://doi.org/10.1038/s41467-019-09404-6.
Judson, R. N., Tremblay, A. M., Knopp, P., White, R. B., Urcia, R., De Bari, C., et al. (2012). The hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. Journal of Cell Science. https://doi.org/10.1242/jcs.109546.
Huang, Z., Hu, J., Pan, J., Wang, Y., Hu, G., Zhou, J., et al. (2016). YAP stabilizes SMAD1 and promotes BMP2-induced neocortical astrocytic differentiation. Development (Cambridge). https://doi.org/10.1242/dev.130658.
Lorthongpanich, C., Thumanu, K., Tangkiettrakul, K., Jiamvoraphong, N., Laowtammathron, C., Damkham, N., et al. (2019). YAP as a key regulator of adipo-osteogenic differentiation in human MSCs. Stem Cell Research and Therapy. https://doi.org/10.1186/s13287-019-1494-4.
Sanderson, S. M., & Locasale, J. W. (2018). Revisiting the Warburg effect: some tumors hold their breath. Cell Metabolism, 28(5), 669–670. https://doi.org/10.1016/j.cmet.2018.10.011.
Altman, B. J., Stine, Z. E., & Dang, C. V. (2016). From Krebs to clinic: glutamine metabolism to cancer therapy. Nature Reviews. Cancer, 16(10), 619–634. https://doi.org/10.1038/nrc.2016.71.
Wu, P.-H., Aroush, D. R.-B., Asnacios, A., Chen, W.-C., Dokukin, M. E., Doss, B. L., et al. (2018). A comparison of methods to assess cell mechanical properties. Nature Methods, 15(7), 491–498. https://doi.org/10.1038/s41592-018-0015-1.
Liu, G. Y., & Sabatini, D. M. (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nature Reviews. Molecular Cell Biology. https://doi.org/10.1038/s41580-019-0199-y.
Park, J. S., Burckhardt, C. J., Lazcano, R., Solis, L. M., Isogai, T., Li, L., et al. (2020). Mechanical regulation of glycolysis via cytoskeleton architecture. Nature, 578(7796), 621–626. https://doi.org/10.1038/s41586-020-1998-1.
Liu, Q.-P., Luo, Q., Deng, B., Ju, Y., & Song, G.-B. (2020). Stiffer matrix accelerates migration of hepatocellular carcinoma cells through enhanced aerobic glycolysis via the MAPK-YAP signaling. Cancers, 12(2). https://doi.org/10.3390/cancers12020490.
Panciera, T., Azzolin, L., Cordenonsi, M., & Piccolo, S. (2017). Mechanobiology of YAP and TAZ in physiology and disease. Nature Reviews Molecular Cell Biology, 18(12), 758–770. https://doi.org/10.1038/nrm.2017.87.
Yin, X., Choudhury, M., Kang, J.-H., Schaefbauer, K. J., Jung, M.-Y., Andrianifahanana, M., et al. (2019). Hexokinase 2 couples glycolysis with the profibrotic actions of TGF-beta. Science Signaling, 12(612). https://doi.org/10.1126/scisignal.aax4067.
Simons, K., & Sampaio, J. L. (2011). Membrane organization and lipid rafts. Cold Spring Harbor Perspectives in Biology, 3(10), a004697. https://doi.org/10.1101/cshperspect.a004697.
Yamada, E. (1955). The fine structure of the gall bladder epithelium of the mouse. The Journal of Biophysical and Biochemical Cytology, 1(5), 445–458. https://doi.org/10.1083/jcb.1.5.445.
Parton, R. G., & Del Pozo, M. A. (2013). Caveolae as plasma membrane sensors, protectors and organizers. Nature Reviews Molecular Cell Biology. https://doi.org/10.1038/nrm3512.
Parton, R. G., Del Pozo, M. A., Vassilopoulos, S., Nabi, I. R., Le Lay, S., Lundmark, R., et al. (2020). Caveolae: the FAQs. Traffic (Copenhagen, Denmark), 21(1), 181–185. https://doi.org/10.1111/tra.12689.
Thomas, C. M., & Smart, E. J. (2008). Caveolae structure and function. Journal of Cellular and Molecular Medicine. https://doi.org/10.1111/j.1582-4934.2008.00295.x.
Parton, R. G. (1996). Caveolae and caveolins. Current Opinion in Cell Biology. https://doi.org/10.1016/S0955-0674(96)80033-0.
Tillu, V. A., Rae, J., Gao, Y., Ariotti, N., Floetenmeyer, M., Kovtun, O., et al. (2019). Cavin1 intrinsically disordered domains are essential for fuzzy electrostatic interactions and caveola formation. bioRxiv, 831149. https://doi.org/10.1101/831149.
Rothberg, K. G., Heuser, J. E., Donzell, W. C., Ying, Y. S., Glenney, J. R., & Anderson, R. G. W. (1992). Caveolin, a protein component of caveolae membrane coats. Cell. https://doi.org/10.1016/0092-8674(92)90143-Z.
Rudick, M., & Anderson, R. G. W. (2002). Multiple functions of caveolin-1. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.R200020200.
Liu, L., Brown, D., McKee, M., LeBrasseur, N. K., Yang, D., Albrecht, K. H., et al. (2008). Deletion of Cavin/PTRF causes global loss of caveolae, dyslipidemia, and glucose intolerance. Cell Metabolism. https://doi.org/10.1016/j.cmet.2008.07.008.
Hill, M. M., Bastiani, M., Luetterforst, R., Kirkham, M., Kirkham, A., Nixon, S. J., et al. (2008). PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell, 132(1), 113–124. https://doi.org/10.1016/j.cell.2007.11.042.
Golani, G., Ariotti, N., Parton, R. G., & Kozlov, M. M. (2019). Membrane curvature and tension control the formation and collapse of caveolar superstructures. Developmental Cell, 48(4), 523–538.e4. https://doi.org/10.1016/j.devcel.2018.12.005.
Ariotti, N., Rae, J., Leneva, N., Ferguson, C., Loo, D., Okano, S., et al. (2015). Molecular characterization of caveolin-induced membrane curvature. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.M115.644336.
Tian, J., Hou, Y., Lu, Q., Wiseman, D. A., Vasconcelos Fonsesca, F., Elms, S., et al. (2010). A novel role for caveolin-1 in regulating endothelial nitric oxide synthase activation in response to H2O2 and shear stress. Free Radical Biology & Medicine, 49(2), 159–170. https://doi.org/10.1016/j.freeradbiomed.2010.03.023.
Mastick, C. C., Brady, M. J., & Saltiel, A. R. (1995). Insulin stimulates the tyrosine phosphorylation of caveolin. Journal of Cell Biology, 129(6), 1523–1531. https://doi.org/10.1083/jcb.129.6.1523.
del Pozo, M. A., Balasubramanian, N., Alderson, N. B., Kiosses, W. B., Grande-García, A., Anderson, R. G. W., & Schwartz, M. A. (2005). Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization. Nature Cell Biology. https://doi.org/10.1038/ncb1293.
Joshi, B., Bastiani, M., Strugnell, S. S., Boscher, C., Parton, R. G., & Nabi, I. R. (2012). Phosphocaveolin-1 is a mechanotransducer that induces caveola biogenesis via Egr1 transcriptional regulation. Journal of Cell Biology, 199(3), 425–435. https://doi.org/10.1083/jcb.201207089.
Goetz, J. G., Minguet, S., Navarro-Lérida, I., Lazcano, J. J., Samaniego, R., Calvo, E., et al. (2011). Biomechanical remodeling of the microenvironment by stromal caveolin-1 favors tumor invasion and metastasis. Cell. https://doi.org/10.1016/j.cell.2011.05.040.
Grande-Garcia, A., Echarri, A., de Rooij, J., Alderson, N. B., Waterman-Storer, C. M., Valdivielso, J. M., & del Pozo, M. A. (2007). Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. The Journal of Cell Biology, 177(4), 683–694. https://doi.org/10.1083/jcb.200701006.
Joshi, B., Strugnell, S. S., Goetz, J. G., Kojic, L. D., Cox, M. E., Griffith, O. L., et al. (2008). Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Research, 68(20), 8210–8220. https://doi.org/10.1158/0008-5472.CAN-08-0343.
Woodman, S. E., Schlegel, A., Cohen, A. W., & Lisanti, M. P. (2002). Mutational analysis identifies a short atypical membrane attachment sequence (KYWFYR) within caveolin-1. Biochemistry. https://doi.org/10.1021/bi0120751.
Dietzen, D. J., Hastings, W. R., & Lublin, D. M. (1995). Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.270.12.6838.
Monier, S., Parton, R. G., Vogel, F., Behlke, J., Henske, A., & Kurzchalia, T. V. (1995). VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.6.7.911.
Muriel, O., Sánchez-Álvarez, M., Strippoli, R., & Del Pozo, M. A. (2018). Role of the endocytosis of caveolae in intracellular signaling and metabolism. Progress in Molecular and Subcellular Biology, 57. https://doi.org/10.1007/978-3-319-96704-2_8.
Okamoto, T., Schlegel, A., Scherer, P. E., & Lisanti, M. P. (1998). Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.273.10.5419.
Schlegel, A., & Lisanti, M. P. (2001). Caveolae and their coat proteins, the caveolins: from electron microscopic novelty to biological launching pad. Journal of Cellular Physiology, 186(3), 329–337. https://doi.org/10.1002/1097-4652(2001)9999:9999<000::AID-JCP1045>3.0.CO;2-0.
Murata, M., Peränen, J., Schreiner, R., Wieland, F., Kurzchalia, T. V., & Simons, K. (1995). VIP21/caveolin is a cholesterol-binding protein. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.92.22.10339.
Schlegel, A., Schwab, R. B., Scherer, P. E., & Lisanti, M. P. (1999). A role for the caveolin scaffolding domain in mediating the membrane attachment of caveolin-1. The caveolin scaffolding domain is both necessary and sufficient for membrane binding in vitro. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.274.32.22660.
Li, S., Song, K. S., & Lisanti, M. P. (1996). Expression and characterization of recombinant caveolin: purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. Journal of Biological Chemistry. https://doi.org/10.1074/jbc.271.1.568.
Krishna, A., & Sengupta, D. (2019). Interplay between membrane curvature and cholesterol: role of palmitoylated caveolin-1. Biophysical Journal. https://doi.org/10.1016/j.bpj.2018.11.3127.
Chang, W. J., Rothberg, K. G., Kamen, B. A., & Anderson, R. G. W. (1992). Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. Journal of Cell Biology. https://doi.org/10.1083/jcb.118.1.63.
Luo, X., Cheng, C., Tan, Z., Li, N., Tang, M., Yang, L., & Cao, Y. (2017). Emerging roles of lipid metabolism in cancer metastasis. Molecular Cancer. https://doi.org/10.1186/s12943-017-0646-3.
Hayer, A., Stoeber, M., Bissig, C., & Helenius, A. (2010). Biogenesis of caveolae: stepwise assembly of large caveolin and cavin complexes. Traffic (Copenhagen, Denmark), 11(3), 361–382. https://doi.org/10.1111/j.1600-0854.2009.01023.x.
Khater, I. M., Liu, Q., Chou, K. C., Hamarneh, G., & Nabi, I. R. (2019). Super-resolution modularity analysis shows polyhedral caveolin-1 oligomers combine to form scaffolds and caveolae. Scientific Reports. https://doi.org/10.1038/s41598-019-46174-z.
Ludwig, A., Howard, G., Mendoza-Topaz, C., Deerinck, T., Mackey, M., Sandin, S., et al. (2013). Molecular composition and ultrastructure of the caveolar coat complex. PLoS Biology, 11(8). https://doi.org/10.1371/journal.pbio.1001640.
Ludwig, A., Nichols, B. J., & Sandin, S. (2016). Architecture of the caveolar coat complex. Journal of Cell Science, 129(16), 3077–3083. https://doi.org/10.1242/jcs.191262.
Stoeber, M., Schellenberger, P., Siebert, C. A., Leyrat, C., Grünewald, K., & Helenius, A. (2016). Model for the architecture of caveolae based on a flexible, net-like assembly of Cavin1 and Caveolin discs. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1073/pnas.1616838113.
Bruno, C., Sotgia, F., Gazzerro, E., Minetti, C., & Lisanti, M. P. (1993). Caveolinopathies. In M. P. Adam, H. H. Ardinger, R. A. Pagon, S. E. Wallace, L. J. H. Bean, K. Stephens, & A. Amemiya (Eds.). Seattle.
Echarri, A., Pavón, D. M., Sánchez, S., García-García, M., Calvo, E., Huerta-López, C., et al. (2019). An Abl-FBP17 mechanosensing system couples local plasma membrane curvature and stress fiber remodeling during mechanoadaptation. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-13782-2.
Echarri, A., & Del Pozo, M. A. (2015). Caveolae—mechanosensitive membrane invaginations linked to actin filaments. Journal of Cell Science, 128(15), 2747–2758. https://doi.org/10.1242/jcs.153940.
Sinha, B., Köster, D., Ruez, R., Gonnord, P., Bastiani, M., Abankwa, D., et al. (2011). Cells respond to mechanical stress by rapid disassembly of caveolae. Cell, 144(3), 402–413. https://doi.org/10.1016/j.cell.2010.12.031.
Tachikawa, M., Morone, N., Senju, Y., Sugiura, T., Hanawa-Suetsugu, K., Mochizuki, A., & Suetsugu, S. (2017). Measurement of caveolin-1 densities in the cell membrane for quantification of caveolar deformation after exposure to hypotonic membrane tension. Scientific Reports, 7(1), 7794. https://doi.org/10.1038/s41598-017-08259-5.
Dewulf, M., Köster, D. V., Sinha, B., Viaris de Lesegno, C., Chambon, V., Bigot, A., et al. (2019). Dystrophy-associated caveolin-3 mutations reveal that caveolae couple IL6/STAT3 signaling with mechanosensing in human muscle cells. Nature Communications, 10(1). https://doi.org/10.1038/s41467-019-09405-5.
Lo, H. P., Nixon, S. J., Hall, T. E., Cowling, B. S., Ferguson, C., Morgan, G. P., et al. (2015). The caveolin-Cavin system plays a conserved and critical role in mechanoprotection of skeletal muscle. Journal of Cell Biology, 210(5), 833–849. https://doi.org/10.1083/jcb.201501046.
Lim, Y. W., Lo, H. P., Ferguson, C., Martel, N., Giacomotto, J., Gomez, G. A., et al. (2017). Caveolae protect notochord cells against catastrophic mechanical failure during development. Current Biology, 27(13), 1968–1981.e7. https://doi.org/10.1016/j.cub.2017.05.067.
Kovtun, O., Tillu, V. A., Jung, W., Leneva, N., Ariotti, N., Chaudhary, N., et al. (2014). Structural insights into the organization of the cavin membrane coat complex. Developmental Cell, 31(4), 405–419. https://doi.org/10.1016/j.devcel.2014.10.002.
Egorov, Y. V., Lang, D., Tyan, L., Turner, D., Lim, E., Piro, Z. D., et al. (2019). Caveolae-mediated activation of mechanosensitive chloride channels in pulmonary veins triggers atrial arrhythmogenesis. Journal of the American Heart Association. https://doi.org/10.1161/JAHA.119.012748.
Kozera, L., White, E., & Calaghan, S. (2009). Caveolae act as membrane reserves which limit mechanosensitive ICl, swell channel activation during swelling in the rat ventricular myocyte. PLoS One, 4(12). https://doi.org/10.1371/journal.pone.0008312.
Ariotti, N., Fernandez-Rojo, M. A., Zhou, Y., Hill, M. M., Rodkey, T. L., Inder, K. L., et al. (2014). Caveolae regulate the nanoscale organization of the plasma membrane to remotely control Ras signaling. Journal of Cell Biology, 204(5), 777–792. https://doi.org/10.1083/jcb.201307055.
Rubin, J., Schwartz, Z., Boyan, B. D., Fan, X., Case, N., Sen, B., et al. (2007). Caveolin-1 knockout mice have increased bone size and stiffness. Journal of Bone and Mineral Research: the Official Journal of the American Society for Bone and Mineral Research, 22(9), 1408–1418. https://doi.org/10.1359/jbmr.070601.
Radel, C., Carlile-Klusacek, M., & Rizzo, V. (2007). Participation of caveolae in beta1 integrin-mediated mechanotransduction. Biochemical and Biophysical Research Communications, 358(2), 626–631. https://doi.org/10.1016/j.bbrc.2007.04.179.
Shi, F., & Sottile, J. (2008). Caveolin-1-dependent beta1 integrin endocytosis is a critical regulator of fibronectin turnover. Journal of Cell Science, 121(Pt 14), 2360–2371. https://doi.org/10.1242/jcs.014977.
Del Pozo, M. A., & Schwartz, M. A. (2007). Rac, membrane heterogeneity, caveolin and regulation of growth by integrins. Trends in Cell Biology. https://doi.org/10.1016/j.tcb.2007.03.001.
Sottile, J., & Chandler, J. (2005). Fibronectin matrix turnover occurs through a caveolin-1-dependent process. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.E04-08-0672.
Boscher, C., & Nabi, I. R. (2013). Galectin-3- and phospho-caveolin-1-dependent outside-in integrin signaling mediates the EGF motogenic response in mammary cancer cells. Molecular Biology of the Cell, 24(13), 2134–2145. https://doi.org/10.1091/mbc.E13-02-0095.
Kozyulina, P. Y., Loskutov, Y. V., Kozyreva, V. K., Rajulapati, A., Ice, R. J., Jones, B. C., & Pugacheva, E. N. (2015). Prometastatic NEDD9 regulates individual cell migration via caveolin-1-dependent trafficking of integrins. Molecular Cancer Research. https://doi.org/10.1158/1541-7786.MCR-14-0353.
Bottcher, R. T., & Fassler, R. (2014). Membrane tension drives ligand-independent integrin signaling. The EMBO Journal, 33(21), 2439–2441. https://doi.org/10.15252/embj.201489886.
Ferraris, G. M. S., Schulte, C., Buttiglione, V., De Lorenzi, V., Piontini, A., Galluzzi, M., et al. (2014). The interaction between uPAR and vitronectin triggers ligand-independent adhesion signalling by integrins. The EMBO Journal. https://doi.org/10.15252/embj.201387611.
Burridge, K., & Wittchen, E. S. (2013). The tension mounts: stress fibers as force-generating mechanotransducers. Journal of Cell Biology. https://doi.org/10.1083/jcb.201210090.
Hayakawa, K., Tatsumi, H., & Sokabe, M. (2011). Actin filaments function as a tension sensor by tension-dependent binding of cofilin to the filament. Journal of Cell Biology. https://doi.org/10.1083/jcb.201102039.
Nevins, A. K., & Thurmond, D. C. (2006). Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. The Journal of Biological Chemistry, 281(28), 18961–18972. https://doi.org/10.1074/jbc.M603604200.
Echarri, A., Muriel, O., Pavón, D. M., Azegrouz, H., Escolar, F., Terrón, M. C., et al. (2012). Caveolar domain organization and trafficking is regulated by Abl kinases and mDia1. Journal of Cell Science, 125(Pt 13), 3097–3113. https://doi.org/10.1242/jcs.090134.
Muriel, O., Echarri, A., Hellriegel, C., Pavón, D. M., Beccari, L., & del Pozo, M. A. (2011). Phosphorylated filamin A regulates actin-linked caveolae dynamics. Journal of Cell Science. https://doi.org/10.1242/jcs.080804.
Mundy, D. I., Machleidt, T., Ying, Y. S., Anderson, R. G. W., & Bloom, G. S. (2002). Dual control of caveolar membrane traffic by microtubules and the actin cytoskeleton. Journal of Cell Science. https://doi.org/10.1242/jcs.00117.
Peng, F., Wu, D., Ingram, A. J., Zhang, B., Gao, B., & Krepinsky, J. C. (2007). RhoA activation in mesangial cells by mechanical strain depends on caveolae and caveolin-1 interaction. Journal of the American Society of Nephrology: JASN, 18(1), 189–198. https://doi.org/10.1681/ASN.2006050498.
Rangel, L., Bernabe-Rubio, M., Fernandez-Barrera, J., Casares-Arias, J., Millan, J., Alonso, M. A., & Correas, I. (2019). Caveolin-1alpha regulates primary cilium length by controlling RhoA GTPase activity. Scientific Reports, 9(1), 1116. https://doi.org/10.1038/s41598-018-38020-5.
Ogata, T., Ueyama, T., Isodono, K., Tagawa, M., Takehara, N., Kawashima, T., et al. (2008). MURC, a muscle-restricted coiled-coil protein that modulates the rho/ROCK pathway, induces cardiac dysfunction and conduction disturbance. Molecular and Cellular Biology. https://doi.org/10.1128/mcb.02186-07.
Kawamura, S., Miyamoto, S., & Brown, J. H. (2003). Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. The Journal of Biological Chemistry, 278(33), 31111–31117. https://doi.org/10.1074/jbc.M300725200.
Galbiati, F., Volonte, D., Liu, J., Capozza, F., Frank, P. G., Zhu, L., et al. (2001). Caveolin-1 expression negatively regulates cell cycle progression by inducing G0/G1 arrest via a p53/p21WAF1/Cip1-dependent mechanism. Molecular Biology of the Cell. https://doi.org/10.1091/mbc.12.8.2229.
Engelman, J. A., Chu, C., Lin, A., Jo, H., Ikezu, T., Okamoto, T., et al. (1998). Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo: a role for the caveolin-scaffolding domain. FEBS Letters. https://doi.org/10.1016/S0014-5793(98)00470-0.
Sunaga, N., Miyajima, K., Suzuki, M., Sato, M., White, M. A., Ramirez, R. D., et al. (2004). Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Research. https://doi.org/10.1158/0008-5472.CAN-03-3941.
Williams, T. M., & Lisanti, M. P. (2005). Caveolin-1 in oncogenic transformation, cancer, and metastasis. American Journal of Physiology - Cell Physiology. https://doi.org/10.1152/ajpcell.00458.2004.
Goetz, J. G., Lajoie, P., Wiseman, S. M., & Nabi, I. R. (2008). Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer and Metastasis Reviews. https://doi.org/10.1007/s10555-008-9160-9.
Martinez-Outschoorn, U. E., Sotgia, F., & Lisanti, M. P. (2015). Caveolae and signalling in cancer. Nature Reviews Cancer. https://doi.org/10.1038/nrc3915.
Hung, K. F., Lin, S. C., Liu, C. J., Chang, C. S., Chang, K. W., & Kao, S. Y. (2003). The biphasic differential expression of the cellular membrane protein, caveolin-1, in oral carcinogenesis. Journal of Oral Pathology and Medicine. https://doi.org/10.1034/j.1600-0714.2003.00185.x.
Pellinen, T., Blom, S., Sánchez, S., Välimäki, K., Mpindi, J.-P., Azegrouz, H., et al. (2018). ITGB1-dependent upregulation of Caveolin-1 switches TGFβ signalling from tumour-suppressive to oncogenic in prostate cancer. Scientific Reports, 8(1). https://doi.org/10.1038/s41598-018-20161-2.
Cerezo, A., Guadamillas, M. C., Goetz, J. G., Sanchez-Perales, S., Klein, E., Assoian, R. K., & del Pozo, M. A. (2009). The absence of caveolin-1 increases proliferation and anchorage- independent growth by a Rac-dependent, Erk-independent mechanism. Molecular and Cellular Biology, 29(18), 5046–5059. https://doi.org/10.1128/mcb.00315-09.
Lamaze, C., & Torrino, S. (2015). Caveolae and cancer: a new mechanical perspective. Biomedical Journal. https://doi.org/10.4103/2319-4170.164229.
Bosch, M., Marí, M., Gross, S. P., Fernández-Checa, J. C., & Pol, A. (2011). Mitochondrial cholesterol: a connection between caveolin, metabolism, and disease. Traffic, 12(11), 1483–1489. https://doi.org/10.1111/j.1600-0854.2011.01259.x.
Alejandro Fernández-Rojo, M., Restall, C., Ferguson, C., Martel, N., Martin, S., Bosch, M., et al. (2012). Caveolin-1 orchestrates the balance between glucose and lipid-dependent energy metabolism: implications for liver regeneration. Hepatology. https://doi.org/10.1002/hep.24810.
Palacios-Ortega, S., Varela-Guruceaga, M., Milagro, F. I., Martínez, J. A., & De Miguel, C. (2014). Expression of caveolin 1 is enhanced by DNA demethylation during adipocyte differentiation. Status of insulin signaling. PLoS ONE. https://doi.org/10.1371/journal.pone.0095100.
Guan, X., Wang, N., Cui, F., Liu, Y., Liu, P., Zhao, J., et al. (2016). Caveolin-1 is essential in the differentiation of human adipose-derived stem cells into hepatocyte-like cells via an MAPK pathway-dependent mechanism. Molecular Medicine Reports. https://doi.org/10.3892/mmr.2015.4743.
Fernandez, M. A., Albor, C., Ingelmo-Torres, M., Nixon, S. J., Ferguson, C., Kurzchalia, T., et al. (2006). Caveolin-1 is essential for liver regeneration. Science, 313(5793), 1628–1632. https://doi.org/10.1126/science.1130773.
Baker, N., & Tuan, R. S. (2013). The less-often-traveled surface of stem cells: caveolin-1 and caveolae in stem cells, tissue repair and regeneration. Stem Cell Research and Therapy. https://doi.org/10.1186/scrt276.
Navab, R., Strumpf, D., To, C, Pasko, E., Kim, K. S., Park, C. J., et al. (2016). Integrin alpha11beta1 regulates cancer stromal stiffness and promotes tumorigenicity and metastasis in non-small cell lung cancer. Oncogene, 35(15), 1899–1908. https://doi.org/10.1038/onc.2015.254.
Alonso-Nocelo, M., Raimondo, T. M., Vining, K. H., Lopez-Lopez, R., de la Fuente, M., & Mooney, D. J. (2018). Matrix stiffness and tumor-associated macrophages modulate epithelial to mesenchymal transition of human adenocarcinoma cells. Biofabrication, 10(3), 35004. https://doi.org/10.1088/1758-5090/aaafbc.
Ciucci, S., Ge, Y., Durán, C., Palladini, A., Jiménez-Jiménez, V., Martínez-Sánchez, L. M., et al. (2017). Enlightening discriminative network functional modules behind principal component analysis separation in differential-omic science studies. Scientific Reports. https://doi.org/10.1038/srep43946.
Hall, B. K., & Gillis, J. A. (2013). Incremental evolution of the neural crest, neural crest cells and neural crest-derived skeletal tissues. Journal of Anatomy. https://doi.org/10.1111/j.1469-7580.2012.01495.x.
Wang, S., Kan, Q., Sun, Y., Han, R., Zhang, G., Peng, T., & Jia, Y. (2013). Caveolin-1 regulates neural differentiation of rat bone mesenchymal stem cells into neurons by modulating notch signaling. International Journal of Developmental Neuroscience. https://doi.org/10.1016/j.ijdevneu.2012.09.004.
Li, Y., Luo, J., Lau, W. M., Zheng, G., Fu, S., Wang, T. T., et al. (2011). Caveolin-1 plays a crucial role in inhibiting neuronal differentiation of neural stem/progenitor cells via VEGF signaling-dependent pathway. PLoS One. https://doi.org/10.1371/journal.pone.0022901.
Hart, P. C., Ratti, B. A., Mao, M., Ansenberger-Fricano, K., Shajahan-Haq, A. N., Tyner, A. L., et al. (2016). Caveolin-1 regulates cancer cell metabolism via scavenging Nrf2 and suppressing MnSOD-driven glycolysis. Oncotarget. https://doi.org/10.18632/ONCOTARGET.5687.
Nwosu, Z. C., Ebert, M. P., Dooley, S., & Meyer, C. (2016). Caveolin-1 in the regulation of cell metabolism: a cancer perspective. Molecular Cancer. https://doi.org/10.1186/s12943-016-0558-7.
Poser, S. W., Otto, O., Arps-Forker, C., Ge, Y., Herbig, M., Andree, C., et al. (2019). Controlling distinct signaling states in cultured cancer cells provides a new platform for drug discovery. FASEB Journal. https://doi.org/10.1096/fj.201802603RR.
Witkiewicz, A. K., Dasgupta, A., Sammons, S., Er, O., Potoczek, M. B., Guiles, F., et al. (2010). Loss of stromal caveolin-1 expression predicts poor clinical outcome in triple negative and basal-like breast cancers. Cancer Biology & Therapy, 10(2), 135–143. https://doi.org/10.4161/cbt.10.2.11983.
Sloan, E. K., Ciocca, D. R., Pouliot, N., Natoli, A., Restall, C., Henderson, M. A., et al. (2009). Stromal cell expression of caveolin-1 predicts outcome in breast cancer. The American Journal of Pathology, 174(6), 2035–2043. https://doi.org/10.2353/ajpath.2009.080924.
Onion, D., Isherwood, M., Shridhar, N., Xenophontos, M., Craze, M. L., Day, L. J., et al. (2018). Multicomponent analysis of the tumour microenvironment reveals low CD8 T cell number, low stromal caveolin-1 and high tenascin-C and their combination as significant prognostic markers in non-small cell lung cancer. Oncotarget, 9(2), 1760–1771. https://doi.org/10.18632/oncotarget.18880.
Gerstenberger, W., Wrage, M., Kettunen, E., Pantel, K., Anttila, S., Steurer, S., & Wikman, H. (2018). Stromal caveolin-1 and caveolin-2 expression in primary tumors and lymph node metastases. Analytical Cellular Pathology (Amsterdam), 2018, 8651790. https://doi.org/10.1155/2018/8651790.
Kamposioras, K., Tsimplouli, C., Verbeke, C., Anthoney, A., Daoukopoulou, A., Papandreou, C. N., et al. (2019). Silencing of caveolin-1 in fibroblasts as opposed to epithelial tumor cells results in increased tumor growth rate and chemoresistance in a human pancreatic cancer model. International Journal of Oncology, 54(2), 537–549. https://doi.org/10.3892/ijo.2018.4640.
Qian, X.-L., Pan, Y.-H., Huang, Q.-Y., Shi, Y.-B., Huang, Q.-Y., Hu, Z.-Z., & Xiong, L.-X. (2019). Caveolin-1: a multifaceted driver of breast cancer progression and its application in clinical treatment. Oncotargets and Therapy, 12, 1539–1552. https://doi.org/10.2147/OTT.S191317.
Eliyatkin, N., Aktas, S., Diniz, G., Ozgur, H. H., Ekin, Z. Y., & Kupelioglu, A. (2018). Expression of stromal caveolin-1 may be a predictor for aggressive behaviour of breast cancer. Pathology Oncology Research, 24(1), 59–65. https://doi.org/10.1007/s12253-017-0212-8.
Neofytou, K., Pikoulis, E., Petrou, A., Agrogiannis, G., Petrides, C., Papakonstandinou, I., et al. (2017). Weak stromal caveolin-1 expression in colorectal liver metastases predicts poor prognosis after hepatectomy for liver-only colorectal metastases. Scientific Reports, 7(1), 2058. https://doi.org/10.1038/s41598-017-02251-9.
Mohammed, D. A., & Helal, D. S. (2017). Prognostic significance of epithelial/stromal caveolin-1 expression in prostatic hyperplasia, high grade prostatic intraepithelial hyperplasia and prostatic carcinoma and its correlation with microvessel density. Journal of the Egyptian National Cancer Institute, 29(1), 25–31. https://doi.org/10.1016/j.jnci.2017.01.002.
Razani, B., Zhang, X. L., Bitzer, M., von Gersdorff, G., Böttinger, E. P., & Lisanti, M. P. (2001). Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor. The Journal of Biological Chemistry, 276(9), 6727–6738. https://doi.org/10.1074/jbc.M008340200.
Couet, J., Sargiacomo, M., & Lisanti, M. P. (1997). Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. The Journal of Biological Chemistry, 272(48), 30429–30438. https://doi.org/10.1074/jbc.272.48.30429.
Yamamoto, M., Toya, Y., Schwencke, C., Lisanti, M. P., Myers, M. G. J., & Ishikawa, Y. (1998). Caveolin is an activator of insulin receptor signaling. The Journal of Biological Chemistry, 273(41), 26962–26968. https://doi.org/10.1074/jbc.273.41.26962.
Schwartz, E. A., Reaven, E., Topper, J. N., & Tsao, P. S. (2005). Transforming growth factor-beta receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. The Biochemical Journal, 390(Pt 1), 199–206. https://doi.org/10.1042/BJ20041182.
Santibanez, J. F., Blanco, F. J., Garrido-Martin, E. M., Sanz-Rodriguez, F., del Pozo, M. A., & Bernabeu, C. (2008). Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovascular Research, 77(4), 791–799. https://doi.org/10.1093/cvr/cvm097.
Strippoli, R., Loureiro, J., Moreno, V., Benedicto, I., Lozano, M. L. P., Barreiro, O., et al. (2015). Caveolin-1 deficiency induces a MEK-ERK1/2-Snail-1-dependent epithelial–mesenchymal transition and fibrosis during peritoneal dialysis. EMBO Molecular Medicine, 7(3), 357. https://doi.org/10.15252/emmm.201570010.
Del Galdo, F., Lisanti, M. P., & Jimenez, S. A. (2008). Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Current Opinion in Rheumatology, 20(6), 713–719. https://doi.org/10.1097/bor.0b013e3283103d27.
Albacete-Albacete, L., Navarro-Lerida, I., Lopez, J. A., Martin-Padura, I., Astudillo, A. M., Van-Der-Heyden, M., … del Pozo, M. A. (2018). ECM deposition is driven by caveolin1-dependent regulation of exosomal biogenesis and cargo sorting. bioRxiv.
He, K., Yan, X., Li, N., Dang, S., Xu, L., Zhao, B., et al. (2015). Internalization of the TGF-beta type I receptor into caveolin-1 and EEA1 double-positive early endosomes. Cell Research, 25(6), 738–752. https://doi.org/10.1038/cr.2015.60.
Di Guglielmo, G. M., Le Roy, C., Goodfellow, A. F., & Wrana, J. L. (2003). Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nature Cell Biology, 5(5), 410–421. https://doi.org/10.1038/ncb975.
World Cancer Report 2014. (2015). International Agency for Research on Cancer (IACR). Editors: Bernard W. Stewart and Christopher P. Wild. https://publications.iarc.fr/_publications/media/download/5839/bc44643f904185d5c8eddb933480b5bc18b21dba.pdf.
Chen, C.-L., Yang, P.-H., Kao, Y.-C., Chen, P.-Y., Chung, C.-L., & Wang, S.-W. (2017). Pentabromophenol suppresses TGF-beta signaling by accelerating degradation of type II TGF-beta receptors via caveolae-mediated endocytosis. Scientific Reports, 7, 43206. https://doi.org/10.1038/srep43206.
Ilha, M., da Silveira Moraes, K., Rohden, F., Martins, L. A. M., Borojevic, R., Lenz, G., et al. (2019). Exogenous expression of caveolin-1 is sufficient for hepatic stellate cell activation. Journal of Cellular Biochemistry, 120(11), 19031–19043. https://doi.org/10.1002/jcb.29226.
Wang, X. M., Zhang, Y., Kim, H. P., Zhou, Z., Feghali-Bostwick, C. A., Liu, F., et al. (2006). Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. The Journal of Experimental Medicine, 203(13), 2895–2906. https://doi.org/10.1084/jem.20061536.
Jung, W., Sierecki, E., Bastiani, M., O’Carroll, A., Alexandrov, K., Rae, J., et al. (2018). Cell-free formation and interactome analysis of caveolae. The Journal of Cell Biology, 217(6), 2141–2165. https://doi.org/10.1083/jcb.201707004.
Hunter, I., & Nixon, G. F. (2006). Spatial compartmentalization of tumor necrosis factor (TNF) receptor 1-dependent signaling pathways in human airway smooth muscle cells. Lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kapp. The Journal of Biological Chemistry, 281(45), 34705–34715. https://doi.org/10.1074/jbc.M605738200.
Liubomirski, Y., Lerrer, S., Meshel, T., Rubinstein-Achiasaf, L., Morein, D., Wiemann, S., et al. (2019). Tumor-stroma-inflammation networks promote pro-metastatic chemokines and aggressiveness characteristics in triple-negative breast cancer. Frontiers in Immunology, 10, 757. https://doi.org/10.3389/fimmu.2019.00757.
Lin, Y., Ryan, J., Lewis, J., Wani, M. A., Lingrel, J. B., & Liu, Z.-G. (2003). TRAF2 exerts its antiapoptotic effect by regulating the expression of Kruppel-like factor LKLF. Molecular and Cellular Biology, 23(16), 5849–5856. https://doi.org/10.1128/mcb.23.16.5849-5856.2003.
Kulshrestha, R., Singh, H., Pandey, A., Mehta, A., Bhardwaj, S., & Jaggi, A. S. (2019). Caveolin-1 as a critical component in the pathogenesis of lung fibrosis of different etiology: evidences and mechanisms. Experimental and Molecular Pathology, 111, 104315. https://doi.org/10.1016/j.yexmp.2019.104315.
Fernandez-Rojo, M. A., & Ramm, G. A. (2016). Caveolin-1 function in liver physiology and disease. Trends in Molecular Medicine, 22(10), 889–904. https://doi.org/10.1016/j.molmed.2016.08.007.
Drab, M., Verkade, P., Elger, M., Kasper, M., Lohn, M., Lauterbach, B., et al. (2001). Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science, 293(5539), 2449–2452. https://doi.org/10.1126/science.1062688.
Kurzchalia, T. V., Dupree, P., & Monier, S. (1994). VIP21-caveolin, a protein of the trans-Golgi network and caveolae. FEBS Letters. https://doi.org/10.1016/0014-5793(94)00466-8.
Del Galdo, F., Sotgia, F., de Almeida, C. J., Jasmin, J.-F., Musick, M., Lisanti, M. P., & Jimenez, S. A. (2008). Decreased expression of caveolin 1 in patients with systemic sclerosis: crucial role in the pathogenesis of tissue fibrosis. Arthritis and Rheumatism, 58(9), 2854–2865. https://doi.org/10.1002/art.23791.
Korennykh, A., & Walter, P. (2012). Structural basis of the unfolded protein response. Annual Review of Cell and Developmental Biology. https://doi.org/10.1146/annurev-cellbio-101011-155826.
Runz, H., Miura, K., Weiss, M., & Pepperkok, R. (2006). Sterols regulate ER-export dynamics of secretory cargo protein ts-O45-G. The EMBO Journal, 25(13), 2953–2965. https://doi.org/10.1038/sj.emboj.7601205.
Shimoda, M., Principe, S., Jackson, H. W., Luga, V., Fang, H., Molyneux, S. D., et al. (2014). Loss of the Timp gene family is sufficient for the acquisition of the CAF-like cell state. Nature Cell Biology, 16(9), 889–901. https://doi.org/10.1038/ncb3021.
Al-Yafeai, Z., Yurdagul, A. J., Peretik, J. M., Alfaidi, M., Murphy, P. A., & Orr, A. W. (2018). Endothelial FN (fibronectin) deposition by alpha5beta1 Integrins drives atherogenic inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology, 38(11), 2601–2614. https://doi.org/10.1161/ATVBAHA.118.311705.
Unlu, G., Levic, D. S., Melville, D. B., & Knapik, E. W. (2014). Trafficking mechanisms of extracellular matrix macromolecules: insights from vertebrate development and human diseases. The International Journal of Biochemistry & Cell Biology, 47, 57–67. https://doi.org/10.1016/j.biocel.2013.11.005.
Annabi, B., Lachambre, M., Bousquet-Gagnon, N., Page, M., Gingras, D., & Beliveau, R. (2001). Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. The Biochemical Journal, 353(Pt 3), 547–553. https://doi.org/10.1042/0264-6021:3530547.
Williams, T. M., Medina, F., Badano, I., Hazan, R. B., Hutchinson, J., Muller, W. J., et al. (2004). Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. The Journal of Biological Chemistry, 279(49), 51630–51646. https://doi.org/10.1074/jbc.M409214200.
Labrecque, L., Nyalendo, C., Langlois, S., Durocher, Y., Roghi, C., Murphy, G., et al. (2004). Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. Journal of Biological Chemistry, 279(50), 52132–52140. https://doi.org/10.1074/jbc.M409617200.
Takayanagi, T., Crawford, K. J., Kobayashi, T., Obama, T., Tsuji, T., Elliott, K. J., et al. (2014). Caveolin 1 is critical for abdominal aortic aneurysm formation induced by angiotensin II and inhibition of lysyl oxidase. Clinical science (London, England: 1979), 126(11), 785–794. https://doi.org/10.1042/CS20130660.
Bonuccelli, G., Whitaker-Menezes, D., Castello-Cros, R., Pavlides, S., Pestell, R. G., Fatatis, A., et al. (2010). The reverse Warburg effect: glycolysis inhibitors prevent the tumor promoting effects of caveolin-1 deficient cancer associated fibroblasts. Cell Cycle (Georgetown, Tex.), 9(10), 1960–1971. https://doi.org/10.4161/cc.9.10.11601.
Rausch, V., & Hansen, C. G. (2020). The hippo pathway, YAP/TAZ, and the plasma membrane. Trends in Cell Biology, 30(1), 32–48. https://doi.org/10.1016/j.tcb.2019.10.005.
Rausch, V., Bostrom, J. R., Park, J., Bravo, I. R., Feng, Y., Hay, D. C., et al. (2019). The hippo pathway regulates caveolae expression and mediates flow response via caveolae. Current Biology: CB, 29(2), 242–255.e6. https://doi.org/10.1016/j.cub.2018.11.066.
Moreno-Vicente, R., Pavón, D. M., Martín-Padura, I., Català-Montoro, M., Díez-Sánchez, A., Quílez-Álvarez, A., et al. (2018). Caveolin-1 modulates mechanotransduction responses to substrate stiffness through actin-dependent control of YAP. Cell Reports, 25(6). https://doi.org/10.1016/j.celrep.2018.10.024.
Tourkina, E., Richard, M., Gööz, P., Bonner, M., Pannu, J., Harley, R., et al. (2008). Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. American Journal of Physiology-Lung Cellular and Molecular Physiology, 294(5), L843–L861. https://doi.org/10.1152/ajplung.00295.2007.
Chen, I. X., Chauhan, V. P., Posada, J., Ng, M. R., Wu, M. W., Adstamongkonkul, P., et al. (2019). Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 116(10), 4558–4566. https://doi.org/10.1073/pnas.1815515116.
Ozdemir, B. C., Pentcheva-Hoang, T., Carstens, J. L., Zheng, X., Wu, C.-C., Simpson, T. R., et al. (2015). Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. United States. https://doi.org/10.1016/j.ccell.2015.11.002.
Acknowledgments
We are grateful to the members of our laboratory and colleagues for useful discussions.
Funding
Research in our laboratory has been supported by grants from the Spanish Ministry of Economy, Industry and Competitiveness (MINECO; SAF2011-25047, SAF2014-51876-R, SAF2017-83130-R, IGP-SO grant MINSEV1512-07-2016, CSD2009-0016 and BFU2016-81912-REDC), and the Worldwide Cancer Research Foundation (no. 15-0404) to M.A.d.P. M.A.d.P is also a member of the Tec4Bio consortium (ref. P2018/NMT4443; “Actividades de I+D entre Grupos de Investigación en Tecnologías,” Comunidad Autónoma de Madrid/FEDER, Spain) and is co-recipient of grants from Fundació La Marató de TV3 (674/C/2013 and 201936). M.A.d.P’s group received funding from the European Union Horizon 2020 research and innovation programme under Marie Sklodowska-Curie grant agreement no. 641639 (BIOPOL ETN), of which V.J-J. was ESR trainee. M.S-A. was recipient of a CNIC IPP fellowship (COFUND programme 2014). The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia, Innovación y Universidades (MCNU), and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lolo, F.N., Jiménez-Jiménez, V., Sánchez-Álvarez, M. et al. Tumor-stroma biomechanical crosstalk: a perspective on the role of caveolin-1 in tumor progression. Cancer Metastasis Rev 39, 485–503 (2020). https://doi.org/10.1007/s10555-020-09900-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-020-09900-y