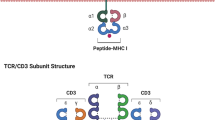
Abstract
The capacity of single-agent therapy with immune checkpoint inhibitors to control solid cancers by unleashing preexisting local antitumor T cell responses has renewed interest in the broader use of T cells as anticancer therapeutics. At the same time, durable responses of refractory B-lineage malignancies to chimeric-receptor engineered T cells illustrate that T cells can be effectively redirected to cancers that lack preexisting tumor antigen-specific T cells, as most typical childhood cancers. This review summarizes strategies by which T cells can be modified to recognize defined antigens, with a focus on chimeric-receptor engineering. We provide an overview of candidate target antigens currently investigated in advanced preclinical and early clinical trials in pediatric malignancies and discuss the prerequisites for an adequate in vivo function of engineered T cells in the microenvironment of solid tumors and intrinsic and extrinsic limitations of current redirected T cell therapies. We further address innovative solutions to recruit therapeutic T cells to tumors, overcome the unreliable and heterogenous expression of most known tumor-associated antigens, and prevent functional inactivation of T cells in the hostile microenvironment of solid childhood tumors.

Similar content being viewed by others
References
Rossig, C., Juergens, H., Schrappe, M., Moericke, A., Henze, G., von Stackelberg, A., et al. (2013). Effective childhood cancer treatment: the impact of large scale clinical trials in Germany and Austria. Pediatric Blood & Cancer, 60(10), 1574–1581.
Stahl, M., Ranft, A., Paulussen, M., Bolling, T., Vieth, V., Bielack, S., et al. (2011). Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatric Blood & Cancer, 57(4), 549–553.
Bielack, S. S., Kempf-Bielack, B., Branscheid, D., Carrle, D., Friedel, G., Helmke, K., et al. (2009). Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. Journal of Clinical Oncology, 27(4), 557–565.
Rossig, C., Juergens, H., & Berdel, W. E. (2011). New targets and targeted drugs for the treatment of cancer: an outlook to pediatric oncology. Pediatric Hematology and Oncology, 28(7), 539–555.
Doebele, R. C., Davis, L. E., Vaishnavi, A., Le, A. T., Estrada-Bernal, A., Keysar, S., et al. (2015). An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discovery, 5(10), 1049–1057.
Brahmer, J., Reckamp, K. L., Baas, P., Crino, L., Eberhardt, W. E., Poddubskaya, E., et al. (2015). Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. The New England Journal of Medicine, 373(2), 123–135.
Kurtulus, S., Madi, A., Escobar, G., Klapholz, M., Nyman, J., Christian, E., et al. (2019). Checkpoint blockade immunotherapy induces dynamic changes in PD-1(-)CD8(+) tumor-infiltrating T cells. Immunity, 50(1), 181–194 e186.
Hui, E., Cheung, J., Zhu, J., Su, X., Taylor, M. J., Wallweber, H. A., et al. (2017). T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science, 355(6332), 1428–1433.
Ott, P. A., Bang, Y. J., Piha-Paul, S. A., Razak, A. R. A., Bennouna, J., Soria, J. C., et al. (2019). T-cell-inflamed gene-expression profile, programmed death ligand 1 expression, and tumor mutational burden predict efficacy in patients treated with pembrolizumab across 20 cancers: KEYNOTE-028. Journal of Clinical Oncology, 37(4), 318–327.
Grobner, S. N., Worst, B. C., Weischenfeldt, J., Buchhalter, I., Kleinheinz, K., Rudneva, V. A., et al. (2018). The landscape of genomic alterations across childhood cancers. Nature, 555(7696), 321–327.
Ma, X., Liu, Y., Liu, Y., Alexandrov, L. B., Edmonson, M. N., Gawad, C., et al. (2018). Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours. Nature, 555(7696), 371–376.
Vakkila, J., Jaffe, R., Michelow, M., & Lotze, M. T. (2006). Pediatric cancers are infiltrated predominantly by macrophages and contain a paucity of dendritic cells: a major nosologic difference with adult tumors. Clinical Cancer Research, 12(7 Pt 1), 2049–2054.
Tawbi, H. A., Burgess, M., Bolejack, V., Van Tine, B. A., Schuetze, S. M., Hu, J., et al. (2017). Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. The Lancet Oncology, 18(11), 1493–1501.
Merchant, M. S., Wright, M., Baird, K., Wexler, L. H., Rodriguez-Galindo, C., Bernstein, D., et al. (2016). Phase I clinical trial of ipilimumab in pediatric patients with advanced solid tumors. Clinical Cancer Research, 22(6), 1364–1370.
Meyer-Wentrup, F., Richter, G., & Burdach, S. (2005). Identification of an immunogenic EWS-FLI1-derived HLA-DR-restricted T helper cell epitope. Pediatric Hematology and Oncology, 22(4), 297–308.
Griffioen, M., Kessler, J. H., Borghi, M., van Soest, R. A., van der Minne, C. E., Nouta, J., et al. (2006). Detection and functional analysis of CD8+ T cells specific for PRAME: a target for T-cell therapy. Clinical Cancer Research, 12(10), 3130–3136.
Schirmer, D., Grunewald, T. G., Klar, R., Schmidt, O., Wohlleber, D., Rubio, R. A., et al. (2016). Transgenic antigen-specific, HLA-A*02:01-allo-restricted cytotoxic T cells recognize tumor-associated target antigen STEAP1 with high specificity. Oncoimmunology, 5(6), e1175795.
Quintarelli, C., Dotti, G., De Angelis, B., Hoyos, V., Mims, M., Luciano, L., et al. (2008). Cytotoxic T lymphocytes directed to the preferentially expressed antigen of melanoma (PRAME) target chronic myeloid leukemia. Blood, 112(5), 1876–1885.
Gerdemann, U., Katari, U., Christin, A. S., Cruz, C. R., Tripic, T., Rousseau, A., et al. (2011). Cytotoxic T lymphocytes simultaneously targeting multiple tumor-associated antigens to treat EBV negative lymphoma. Molecular Therapy, 19(12), 2258–2268.
Altvater, B., Kailayangiri, S., Theimann, N., Ahlmann, M., Farwick, N., Chen, C., et al. (2014). Common Ewing sarcoma-associated antigens fail to induce natural T cell responses in both patients and healthy individuals. Cancer Immunology, Immunotherapy, 63(10), 1047–1060.
Morgan, R. A., Dudley, M. E., Wunderlich, J. R., Hughes, M. S., Yang, J. C., Sherry, R. M., et al. (2006). Cancer regression in patients after transfer of genetically engineered lymphocytes. Science, 314(5796), 126–129.
Robbins, P. F., Li, Y. F., El-Gamil, M., Zhao, Y., Wargo, J. A., Zheng, Z., et al. (2008). Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. Journal of Immunology, 180(9), 6116–6131.
D'Angelo, S. P., Melchiori, L., Merchant, M. S., Bernstein, D., Glod, J., Kaplan, R., et al. (2018). Antitumor activity associated with prolonged persistence of adoptively transferred NY-ESO-1 (c259)T cells in synovial sarcoma. Cancer Discovery, 8(8), 944–957.
Raffaghello, L., Prigione, I., Bocca, P., Morandi, F., Camoriano, M., Gambini, C., et al. (2005). Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene, 24(29), 4634–4644.
Linette, G. P., Stadtmauer, E. A., Maus, M. V., Rapoport, A. P., Levine, B. L., Emery, L., et al. (2013). Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood, 122(6), 863–871.
Mastaglio, S., Genovese, P., Magnani, Z., Ruggiero, E., Landoni, E., Camisa, B., et al. (2017). NY-ESO-1 TCR single edited stem and central memory T cells to treat multiple myeloma without graft-versus-host disease. Blood, 130(5), 606–618.
Bargou, R., Leo, E., Zugmaier, G., Klinger, M., Goebeler, M., Knop, S., et al. (2008). Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science, 321(5891), 974–977.
von Stackelberg, A., Locatelli, F., Zugmaier, G., Handgretinger, R., Trippett, T. M., Rizzari, C., et al. (2016). Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Journal of Clinical Oncology, 34(36), 4381–4389.
Ahmed, M., Cheng, M., Cheung, I. Y., & Cheung, N. K. (2015). Human derived dimerization tag enhances tumor killing potency of a T-cell engaging bispecific antibody. Oncoimmunology, 4(4), e989776.
Finney, H. M., Lawson, A. D., Bebbington, C. R., & Weir, A. N. (1998). Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. Journal of Immunology, 161(6), 2791–2797.
Imai, C., Mihara, K., Andreansky, M., Nicholson, I. C., Pui, C. H., Geiger, T. L., et al. (2004). Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia, 18(4), 676–684.
Eshhar, Z., Waks, T., Gross, G., & Schindler, D. G. (1993). Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America, 90(2), 720–724.
Pule, M. A., Savoldo, B., Myers, G. D., Rossig, C., Russell, H. V., Dotti, G., et al. (2008). Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Medicine, 14(11), 1264–1270.
Turtle, C. J., Hanafi, L. A., Berger, C., Gooley, T. A., Cherian, S., Hudecek, M., et al. (2016). CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. The Journal of Clinical Investigation, 126(6), 2123–2138.
Maude, S. L., Laetsch, T. W., Buechner, J., Rives, S., Boyer, M., Bittencourt, H., et al. (2018). Tisagenlecleucel in children and young adults with B-Cell lymphoblastic leukemia. The New England Journal of Medicine, 378(5), 439–448.
Lee, D. W., Kochenderfer, J. N., Stetler-Stevenson, M., Cui, Y. K., Delbrook, C., Feldman, S. A., et al. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: A phase 1 dose-escalation trial. Lancet, 385(9967), 517–528.
Gardner, R. A., Finney, O., Annesley, C., Brakke, H., Summers, C., Leger, K., et al. (2017). Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood, 129(25), 3322–3331.
Neelapu, S. S., Locke, F. L., Bartlett, N. L., Lekakis, L. J., Miklos, D. B., Jacobson, C. A., et al. (2017). Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. The New England Journal of Medicine, 377(26), 2531–2544.
Heczey, A., Louis, C. U., Savoldo, B., Dakhova, O., Durett, A., Grilley, B., et al. (2017). CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Molecular Therapy, 25(9), 2214–2224.
Louis, C. U., Savoldo, B., Dotti, G., Pule, M., Yvon, E., Myers, G. D., et al. (2011). Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood, 118(23), 6050–6056.
Straathof, K., Flutter, B., Wallace, R., Thomas, S., Cheung, G., Collura, A., et al. (2018). A Cancer Research UK phase I trial of anti-GD2 chimeric antigen receptor (CAR) transduced T-cells (1RG-CART) in patients with relapsed or refractory neuroblastoma. Cancer Research, 78(13 Supplement), CT145.
Ahmed, N., Brawley, V. S., Hegde, M., Robertson, C., Ghazi, A., Gerken, C., et al. (2015). Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. Journal of Clinical Oncology, 33(15), 1688–1696.
Ahmed, N., Brawley, V., Hegde, M., Bielamowicz, K., Kalra, M., Landi, D., et al. (2017). HER2-specific chimeric antigen receptor-modified virus-specific T cells for progressive glioblastoma: a phase 1 dose-escalation trial. JAMA Oncology, 3(8), 1094–1101.
Sotillo, E., Barrett, D. M., Black, K. L., Bagashev, A., Oldridge, D., Wu, G., et al. (2015). Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discovery, 5(12), 1282–1295.
Wu, Z. L., Schwartz, E., Seeger, R., & Ladisch, S. (1986). Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Research, 46(1), 440–443.
Schulz, G., Cheresh, D. A., Varki, N. M., Yu, A., Staffileno, L. K., & Reisfeld, R. A. (1984). Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer Research, 44(12), 5914–5920.
Rossig, C., Bollard, C. M., Nuchtern, J. G., Rooney, C. M., & Brenner, M. K. (2002). Epstein-Barr virus-specific human T lymphocytes expressing antitumor chimeric T-cell receptors: potential for improved immunotherapy. Blood, 99(6), 2009–2016.
Sorkin, L. S., Otto, M., Baldwin III, W. M., Vail, E., Gillies, S. D., Handgretinger, R., et al. (2010). Anti-GD(2) with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain, 149(1), 135–142.
Richman, S. A., Nunez-Cruz, S., Moghimi, B., Li, L. Z., Gershenson, Z. T., Mourelatos, Z., et al. (2018). High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunology Research, 6(1), 36–46.
Andersch, L., Radke, J., Klaus, A., Schwiebert, S., Winkler, A., Schumann, E., et al. (2019). CD171- and GD2-specific CAR-T cells potently target retinoblastoma cells in preclinical in vitro testing. BMC Cancer, 19(1), 895.
Portoukalian, J., David, M. J., Gain, P., & Richard, M. (1993). Shedding of GD2 ganglioside in patients with retinoblastoma. International Journal of Cancer, 53(6), 948–951.
Mount, C. W., Majzner, R. G., Sundaresh, S., Arnold, E. P., Kadapakkam, M., Haile, S., et al. (2018). Potent antitumor efficacy of anti-GD2 CAR T cells in H3-K27M(+) diffuse midline gliomas. Nature Medicine, 24(5), 572–579.
Dobrenkov, K., Ostrovnaya, I., Gu, J., Cheung, I. Y., & Cheung, N. K. (2016). Oncotargets GD2 and GD3 are highly expressed in sarcomas of children, adolescents, and young adults. Pediatric Blood & Cancer, 63(10), 1780–1785.
Kailayangiri, S., Altvater, B., Spurny, C., Jamitzky, S., Schelhaas, S., Jacobs, A. H., et al. (2017). Targeting Ewing sarcoma with activated and GD2-specific chimeric antigen receptor-engineered human NK cells induces upregulation of immune-inhibitory HLA-G. Oncoimmunology, 6(1), e1250050.
Long, A. H., Highfill, S. L., Cui, Y., Smith, J. P., Walker, A. J., Ramakrishna, S., et al. (2016). Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunology Research, 4(10), 869–880.
Roth, M., Linkowski, M., Tarim, J., Piperdi, S., Sowers, R., Geller, D., et al. (2014). Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer, 120(4), 548–554.
Modak, S., Gerald, W., & Cheung, N. K. (2002). Disialoganglioside GD2 and a novel tumor antigen: potential targets for immunotherapy of desmoplastic small round cell tumor. Medical and Pediatric Oncology, 39(6), 547–551.
Geiser, M., Schultz, D., Le Cardinal, A., Voshol, H., & Garcia-Echeverria, C. (1999). Identification of the human melanoma-associated chondroitin sulfate proteoglycan antigen epitope recognized by the antitumor monoclonal antibody 763.74 from a peptide phage library. Cancer Research, 59(4), 905–910.
Svendsen, A., Verhoeff, J. J., Immervoll, H., Brogger, J. C., Kmiecik, J., Poli, A., et al. (2011). Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathologica, 122(4), 495–510.
Pellegatta, S., Savoldo, B., Di Ianni, N., Corbetta, C., Chen, Y., Patane, M., et al. (2018). Constitutive and TNFalpha-inducible expression of chondroitin sulfate proteoglycan 4 in glioblastoma and neurospheres: Implications for CAR-T cell therapy. Science Translational Medicine, 10(430).
Brehm, H., Niesen, J., Mladenov, R., Stein, C., Pardo, A., Fey, G., et al. (2014). A CSPG4-specific immunotoxin kills rhabdomyosarcoma cells and binds to primary tumor tissues. Cancer Letters, 352(2), 228–235.
Wang, X., Katayama, A., Wang, Y., Yu, L., Favoino, E., Sakakura, K., et al. (2011). Functional characterization of an scFv-Fc antibody that immunotherapeutically targets the common cancer cell surface proteoglycan CSPG4. Cancer Research, 71(24), 7410–7422.
Geldres, C., Savoldo, B., Hoyos, V., Caruana, I., Zhang, M., Yvon, E., et al. (2014). T lymphocytes redirected against the chondroitin sulfate proteoglycan-4 control the growth of multiple solid tumors both in vitro and in vivo. Clinical Cancer Research, 20(4), 962–971.
Wang, J., Svendsen, A., Kmiecik, J., Immervoll, H., Skaftnesmo, K. O., Planaguma, J., et al. (2011). Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS One, 6(7), e23062.
Beard, R. E., Zheng, Z., Lagisetty, K. H., Burns, W. R., Tran, E., Hewitt, S. M., et al. (2014). Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. Journal for Immunotherapy of Cancer, 2, 25.
Prieto, C., Lopez-Millan, B., Roca-Ho, H., Stam, R. W., Romero-Moya, D., Rodriguez-Baena, F. J., et al. (2018). Correction: NG2 antigen is involved in leukemia invasiveness and central nervous system infiltration in MLL-rearranged infant B-ALL. Leukemia, 32(10), 2306.
Bosse, K. R., Raman, P., Zhu, Z., Lane, M., Martinez, D., Heitzeneder, S., et al. (2017). Identification of GPC2 as an oncoprotein and candidate immunotherapeutic target in high-risk neuroblastoma. Cancer Cell, 32(3), 295–309 e212.
Li, N., Fu, H., Hewitt, S. M., Dimitrov, D. S., & Ho, M. (2017). Therapeutically targeting glypican-2 via single-domain antibody-based chimeric antigen receptors and immunotoxins in neuroblastoma. Proceedings of the National Academy of Sciences of the United States of America, 114(32), E6623–E6631.
Scotlandi, K., Manara, M. C., Hattinger, C. M., Benini, S., Perdichizzi, S., Pasello, M., et al. (2005). Prognostic and therapeutic relevance of HER2 expression in osteosarcoma and Ewing’s sarcoma. European Journal of Cancer, 41(9), 1349–1361.
Ahmed, N., Salsman, V. S., Yvon, E., Louis, C. U., Perlaky, L., Wels, W. S., et al. (2009). Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Molecular Therapy, 17(10), 1779–1787.
Gorlick, R., Huvos, A. G., Heller, G., Aledo, A., Beardsley, G. P., Healey, J. H., et al. (1999). Expression of HER2/erbB-2 correlates with survival in osteosarcoma. Journal of Clinical Oncology, 17(9), 2781–2788.
Ganti, R., Skapek, S. X., Zhang, J., Fuller, C. E., Wu, J., Billups, C. A., et al. (2006). Expression and genomic status of EGFR and ErbB-2 in alveolar and embryonal rhabdomyosarcoma. Modern Pathology, 19(9), 1213–1220.
Ahmed, N., Ratnayake, M., Savoldo, B., Perlaky, L., Dotti, G., Wels, W. S., et al. (2007). Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Research, 67(12), 5957–5964.
Morgan, R. A., Yang, J. C., Kitano, M., Dudley, M. E., Laurencot, C. M., & Rosenberg, S. A. (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular Therapy, 18(4), 843–851.
Navai, S. A., Derenzo, C., Joseph, S. K., Sanber, K., Byrd, T., Zhang, H., et al. (2019). Administration of HER2-CAR T cells after lymphodepletion safely improves T cell expansion and induces clinical responses in patients with advanced sarcomas. Cancer Research, 79(13 Supplement), LB-147.
Padfield, E., Ellis, H. P., & Kurian, K. M. (2015). Current therapeutic advances targeting EGFR and EGFRvIII in glioblastoma. Frontiers in Oncology, 5, 5.
O'Rourke, D. M., Nasrallah, M. P., Desai, A., Melenhorst, J. J., Mansfield, K., Morrissette, J. J. D., et al. (2017). A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Science Translational Medicine, 9(399).
Gan, H. K., Burgess, A. W., Clayton, A. H., & Scott, A. M. (2012). Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Research, 72(12), 2924–2930.
Goff, S. L., Morgan, R. A., Yang, J. C., Sherry, R. M., Robbins, P. F., Restifo, N. P., et al. (2019). Pilot trial of adoptive transfer of chimeric antigen receptor-transduced T cells targeting EGFRvIII in patients with glioblastoma. Journal of Immunotherapy, 42(4), 126–135.
Debinski, W., Gibo, D. M., Hulet, S. W., Connor, J. R., & Gillespie, G. Y. (1999). Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clinical Cancer Research, 5(5), 985–990.
Newman, J. P., Wang, G. Y., Arima, K., Guan, S. P., Waters, M. R., Cavenee, W. K., et al. (2017). Interleukin-13 receptor alpha 2 cooperates with EGFRvIII signaling to promote glioblastoma multiforme. Nature Communications, 8(1), 1913.
Loftus, R. W., Koff, M. D., Brown, J. R., Patel, H. M., Jensen, J. T., Reddy, S., et al. (2015). The dynamics of Enterococcus transmission from bacterial reservoirs commonly encountered by anesthesia providers. Anesthesia and Analgesia, 120(4), 827–836.
Keu, K. V., Witney, T. H., Yaghoubi, S., Rosenberg, J., Kurien, A., Magnusson, R., et al. (2017). Reporter gene imaging of targeted T cell immunotherapy in recurrent glioma. Science Translational Medicine, 9(373).
Brown, C. E., Alizadeh, D., Starr, R., Weng, L., Wagner, J. R., Naranjo, A., et al. (2016). Regression of glioblastoma after chimeric antigen receptor T-cell therapy. The New England Journal of Medicine, 375(26), 2561–2569.
Passoni, L., Longo, L., Collini, P., Coluccia, A. M., Bozzi, F., Podda, M., et al. (2009). Mutation-independent anaplastic lymphoma kinase overexpression in poor prognosis neuroblastoma patients. Cancer Research, 69(18), 7338–7346.
Mosse, Y. P. (2016). Anaplastic lymphoma kinase as a cancer target in pediatric malignancies. Clinical Cancer Research, 22(3), 546–552.
Walker, A. J., Majzner, R. G., Zhang, L., Wanhainen, K., Long, A. H., Nguyen, S. M., et al. (2017). Tumor antigen and receptor densities regulate efficacy of a chimeric antigen receptor targeting anaplastic lymphoma kinase. Molecular Therapy, 25(9), 2189–2201.
Zhang, W., Xu, Y., Zhang, L., Wang, S., Yin, B., Zhao, S., et al. (2018). Synergistic effects of TGFbeta2, WNT9a, and FGFR4 signals attenuate satellite cell differentiation during skeletal muscle development. Aging Cell, 17(4), e12788.
Khan, J., Wei, J. S., Ringner, M., Saal, L. H., Ladanyi, M., Westermann, F., et al. (2001). Classification and diagnostic prediction of cancers using gene expression profiling and artificial neural networks. Nature Medicine, 7(6), 673–679.
Shern, J. F., Chen, L., Chmielecki, J., Wei, J. S., Patidar, R., Rosenberg, M., et al. (2014). Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discovery, 4(2), 216–231.
Taylor, J. G. T., Cheuk, A. T., Tsang, P. S., Chung, J. Y., Song, Y. K., Desai, K., et al. (2009). Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. The Journal of Clinical Investigation, 119(11), 3395–3407.
McKinnon, T., Venier, R., Yohe, M., Sindiri, S., Gryder, B. E., Shern, J. F., et al. (2018). Functional screening of FGFR4-driven tumorigenesis identifies PI3K/mTOR inhibition as a therapeutic strategy in rhabdomyosarcoma. Oncogene, 37(20), 2630–2644.
Shivaprasad, N., Xiong, Y., Yohe, M., Schneider, D., Shern, J., Baskar, S., et al. (2016). Developing FGFR4 chimeric antigen receptor CAR T cell therapy against rhabdomyosarcoma. Molecular Therapy, 24(1), S257–S258.
Husain, B., Ramani, S.R., Chiang, E., Lehoux, I., Paduchuri, S., Arena, T.A., et al. (2019). A platform for extracellular interactome discovery identifies novel functional binding partners for the immune receptors B7-H3/CD276 and PVR/CD155. Molecular & Cellular Proteomics, Jul 15.
Modak, S., Kramer, K., Gultekin, S. H., Guo, H. F., & Cheung, N. K. (2001). Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Research, 61(10), 4048–4054.
Ahmed, M., Cheng, M., Zhao, Q., Goldgur, Y., Cheal, S. M., Guo, H. F., et al. (2015). Humanized affinity-matured monoclonal antibody 8H9 has potent antitumor activity and binds to FG loop of tumor antigen B7-H3. The Journal of Biological Chemistry, 290(50), 30018–30029.
Castriconi, R., Dondero, A., Augugliaro, R., Cantoni, C., Carnemolla, B., Sementa, A. R., et al. (2004). Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proceedings of the National Academy of Sciences of the United States of America, 101(34), 12640–12645.
Seaman, S., Zhu, Z., Saha, S., Zhang, X. M., Yang, M. Y., Hilton, M. B., et al. (2017). Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell, 31(4), 501–515 e508.
Majzner, R. G., Theruvath, J. L., Nellan, A., Heitzeneder, S., Cui, Y., Mount, C. W., et al. (2019). CAR T cells targeting B7-H3, a pan-cancer antigen, demonstrate potent preclinical activity against pediatric solid tumors and prain tumors. Clinical Cancer Research, 25(8), 2560–2574.
Zhou, Z., Luther, N., Ibrahim, G. M., Hawkins, C., Vibhakar, R., Handler, M. H., et al. (2013). B7-H3, a potential therapeutic target, is expressed in diffuse intrinsic pontine glioma. Journal of Neuro-Oncology, 111(3), 257–264.
Wang, L., Zhang, Q., Chen, W., Shan, B., Ding, Y., Zhang, G., et al. (2013). B7-H3 is overexpressed in patients suffering osteosarcoma and associated with tumor aggressiveness and metastasis. PLoS One, 8(8), e70689.
Loo, D., Alderson, R. F., Chen, F. Z., Huang, L., Zhang, W., Gorlatov, S., et al. (2012). Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clinical Cancer Research, 18(14), 3834–3845.
Kramer, K., Kushner, B. H., Modak, S., Pandit-Taskar, N., Smith-Jones, P., Zanzonico, P., et al. (2010). Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. Journal of Neuro-Oncology, 97(3), 409–418.
Du, H., Hirabayashi, K., Ahn, S., Kren, N. P., Montgomery, S. A., Wang, X., et al. (2019). Antitumor responses in the absence of toxicity in solid tumors by targeting B7-H3 via chimeric antigen receptor T cells. Cancer Cell, 35(2), 221–237 e228.
Nehama, D., Di Ianni, N., Musio, S., Du, H., Patane, M., Pollo, B., et al. (2019). B7-H3-redirected chimeric antigen receptor T cells target glioblastoma and neurospheres. EBioMedicine, 47, 33–43.
Watanabe, K., Terakura, S., Martens, A. C., van Meerten, T., Uchiyama, S., Imai, M., et al. (2015). Target antigen density governs the efficacy of anti-CD20-CD28-CD3 zeta chimeric antigen receptor-modified effector CD8+ T cells. Journal of Immunology, 194(3), 911–920.
Hombach, A. A., Gorgens, A., Chmielewski, M., Murke, F., Kimpel, J., Giebel, B., et al. (2016). Superior therapeutic index in lymphoma therapy: CD30(+) CD34(+) hematopoietic stem cells resist a chimeric antigen receptor T-cell attack. Molecular Therapy, 24(8), 1423–1434.
Fry, T. J., Shah, N. N., Orentas, R. J., Stetler-Stevenson, M., Yuan, C. M., Ramakrishna, S., et al. (2018). CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nature Medicine, 24(1), 20–28.
Kailayangiri, S., Altvater, B., Lesch, S., Balbach, S., Gottlich, C., Kuhnemundt, J., et al. (2019). EZH2 inhibition in Ewing sarcoma upregulates GD2 expression for targeting with gene-modified T cells. Molecular Therapy, 27(5), 933–946.
Ramakrishna, S., Highfill, S. L., Walsh, Z., Nguyen, S. M., Lei, H., Shern, J. F., et al. (2019). Modulation of target antigen density improves CAR T-cell functionality and persistence. Clinical Cancer Research, 25(17), 5329–5341.
Pont, M.J., Hill, T., Cole, G.O., Abbott, J.J., Kelliher, J., Salter, A.I., et al. (2019). gamma-secretase inhibition increases efficacy of BCMA-specific chimeric antigen receptor T cells in multiple myeloma. Blood, Sep 26.
Ruella, M., Barrett, D. M., Kenderian, S. S., Shestova, O., Hofmann, T. J., Perazzelli, J., et al. (2016). Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. The Journal of Clinical Investigation, 126(10), 3814–3826.
Sukumaran, S., Watanabe, N., Bajgain, P., Raja, K., Mohammed, S., Fisher, W. E., et al. (2018). Enhancing the potency and specificity of engineered T cells for cancer treatment. Cancer Discovery, 8(8), 972–987.
Grada, Z., Hegde, M., Byrd, T., Shaffer, D. R., Ghazi, A., Brawley, V. S., et al. (2013). TanCAR: a novel bispecific chimeric antigen receptor for cancer immunotherapy. Molecular Therapy--Nucleic Acids, 2, e105.
Hegde, M., Mukherjee, M., Grada, Z., Pignata, A., Landi, D., Navai, S. A., et al. (2016). Tandem CAR T cells targeting HER2 and IL13Ralpha2 mitigate tumor antigen escape. The Journal of Clinical Investigation, 126(8), 3036–3052.
Ahn, S., Li, J., Sun, C., Gao, K., Hirabayashi, K., Li, H., et al. (2019). Cancer immunotherapy with T cells carrying bispecific receptors that mimic antibodies. Cancer Immunology Research, 7(5), 773–783.
Choi, B. D., Yu, X., Castano, A. P., Bouffard, A. A., Schmidts, A., Larson, R. C., et al. (2019). CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nature Biotechnology, 37(9), 1049–1058.
Lee, Y. G., Marks, I., Srinivasarao, M., Kanduluru, A. K., Mahalingam, S. M., Liu, X., et al. (2019). Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors. Cancer Research, 79(2), 387–396.
Ma, J. S., Kim, J. Y., Kazane, S. A., Choi, S. H., Yun, H. Y., Kim, M. S., et al. (2016). Versatile strategy for controlling the specificity and activity of engineered T cells. Proceedings of the National Academy of Sciences of the United States of America, 113(4), E450–E458.
Urbanska, K., Lanitis, E., Poussin, M., Lynn, R. C., Gavin, B. P., Kelderman, S., et al. (2012). A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Research, 72(7), 1844–1852.
Cartellieri, M., Feldmann, A., Koristka, S., Arndt, C., Loff, S., Ehninger, A., et al. (2016). Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer Journal, 6(8), e458.
Mitwasi, N., Feldmann, A., Bergmann, R., Berndt, N., Arndt, C., Koristka, S., et al. (2017). Development of novel target modules for retargeting of UniCAR T cells to GD2 positive tumor cells. Oncotarget, 8(65), 108584–108603.
Roybal, K. T., Williams, J. Z., Morsut, L., Rupp, L. J., Kolinko, I., Choe, J. H., et al. (2016). Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell, 167(2), 419–432 e416.
Srivastava, S., Salter, A. I., Liggitt, D., Yechan-Gunja, S., Sarvothama, M., Cooper, K., et al. (2019). Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated roxicity to normal tissues and enables selective tumor targeting. Cancer Cell, 35(3), 489–503 e488.
Caruana, I., Savoldo, B., Hoyos, V., Weber, G., Liu, H., Kim, E. S., et al. (2015). Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nature Medicine, 21(5), 524–529.
Binnewies, M., Roberts, E. W., Kersten, K., Chan, V., Fearon, D. F., Merad, M., et al. (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine, 24(5), 541–550.
Parihar, R., Rivas, C., Huynh, M., Omer, B., Lapteva, N., Metelitsa, L. S., et al. (2019). NK cells expressing a chimeric activating receptor eliminate MDSCs and rescue impaired CAR-T cell activity against solid tumors. Cancer Immunology Research, 7(3), 363–375.
Chang, C. H., Qiu, J., O'Sullivan, D., Buck, M. D., Noguchi, T., Curtis, J. D., et al. (2015). Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell, 162(6), 1229–1241.
Turtle, C. J., Hanafi, L. A., Berger, C., Hudecek, M., Pender, B., Robinson, E., et al. (2016). Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Science Translational Medicine, 8(355), 355ra116.
Chmielewski, M., Kopecky, C., Hombach, A. A., & Abken, H. (2011). IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Research, 71(17), 5697–5706.
Chinnasamy, D., Yu, Z., Kerkar, S. P., Zhang, L., Morgan, R. A., Restifo, N. P., et al. (2012). Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clinical Cancer Research, 18(6), 1672–1683.
Zhang, L., Morgan, R. A., Beane, J. D., Zheng, Z., Dudley, M. E., Kassim, S. H., et al. (2015). Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clinical Cancer Research, 21(10), 2278–2288.
Robertson, M. J., Kirkwood, J. M., Logan, T. F., Koch, K. M., Kathman, S., Kirby, L. C., et al. (2008). A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clinical Cancer Research, 14(11), 3462–3469.
Chmielewski, M., & Abken, H. (2017). CAR T cells releasing IL-18 convert to T-Bet(high) FoxO1(low) effectors that exhibit augmented activity against advanced solid tumors. Cell Reports, 21(11), 3205–3219.
Hu, B., Ren, J., Luo, Y., Keith, B., Young, R. M., Scholler, J., et al. (2017). Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Reports, 20(13), 3025–3033.
Avanzi, M. P., Yeku, O., Li, X., Wijewarnasuriya, D. P., van Leeuwen, D. G., Cheung, K., et al. (2018). Engineered tumor-targeted T vells mediate enhanced anti-tumor efficacy both directly and through activation of the endogenous immune system. Cell Reports, 23(7), 2130–2141.
Chen, Y., Sun, C., Landoni, E., Metelitsa, L., Dotti, G., & Savoldo, B. (2019). Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clinical Cancer Research, 25(9), 2915–2924.
Landmeier, S., Altvater, B., Pscherer, S., Eing, B. R., Kuehn, J., Rooney, C. M., et al. (2007). Gene-engineered varicella-zoster virus reactive CD4+ cytotoxic T cells exert tumor-specific effector function. Cancer Research, 67(17), 8335–8343.
Tanaka, M., Tashiro, H., Omer, B., Lapteva, N., Ando, J., Ngo, M., et al. (2017). Vaccination targeting native receptors to enhance the function and proliferation of chimeric antigen receptor (CAR)-modified T cells. Clinical Cancer Research.
Rossig, C., Pule, M., Altvater, B., Saiagh, S., Wright, G., Ghorashian, S., et al. (2017). Vaccination to improve the persistence of CD19CAR gene-modified T cells in relapsed pediatric acute lymphoblastic leukemia. Leukemia, 31(5), 1087–1095.
Ma, L., Dichwalkar, T., Chang, J. Y. H., Cossette, B., Garafola, D., Zhang, A. Q., et al. (2019). Enhanced CAR-T cell activity against solid tumors by vaccine boosting through the chimeric receptor. Science, 365(6449), 162–168.
VanSeggelen, H., Tantalo, D. G., Afsahi, A., Hammill, J. A., & Bramson, J. L. (2015). Chimeric antigen receptor-engineered T cells as oncolytic virus carriers. Molecular Therapy–Oncolytics, 2, 15014.
Tanoue, K., Rosewell Shaw, A., Watanabe, N., Porter, C., Rana, B., Gottschalk, S., et al. (2017). Armed oncolytic adenovirus-expressing PD-L1 mini-body enhances antitumor effects of chimeric antigen receptor T cells in solid tumors. Cancer Research, 77(8), 2040–2051.
Jacoby, E., Bielorai, B., Avigdor, A., Itzhaki, O., Hutt, D., Nussboim, V., et al. (2018). Locally produced CD19 CAR T cells leading to clinical remissions in medullary and extramedullary relapsed acute lymphoblastic leukemia. American Journal of Hematology, 93(12), 1485–1492.
Schuster, S. J., Svoboda, J., Chong, E. A., Nasta, S. D., Mato, A. R., Anak, O., et al. (2017). Chimeric antigen receptor T cells in refractory B-cell lymphomas. The New England Journal of Medicine, 377(26), 2545–2554.
Kawalekar, O. U., O'Connor, R. S., Fraietta, J. A., Guo, L., McGettigan, S. E., Posey Jr., A. D., et al. (2016). Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity, 44(2), 380–390.
Shum, T., Omer, B., Tashiro, H., Kruse, R. L., Wagner, D. L., Parikh, K., et al. (2017). Constitutive signaling from an engineered IL7 receptor promotes durable tumor elimination by tumor-redirected T cells. Cancer Discovery, 7(11), 1238–1247.
Davenport, A. J., Cross, R. S., Watson, K. A., Liao, Y., Shi, W., Prince, H. M., et al. (2018). Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 115(9), E2068–E2076.
Long, A. H., Haso, W. M., Shern, J. F., Wanhainen, K. M., Murgai, M., Ingaramo, M., et al. (2015). 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature Medicine, 21(6), 581–590.
Gomes-Silva, D., Mukherjee, M., Srinivasan, M., Krenciute, G., Dakhova, O., Zheng, Y., et al. (2017). Tonic 4-1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Reports, 21(1), 17–26.
Fisher, J., Sharma, R., Don, D. W., Barisa, M., Hurtado, M. O., Abramowski, P., et al. (2019). Engineering gammadeltaT cells limits tonic signaling associated with chimeric antigen receptors. Science Signaling, 12(598).
Watanabe, N., Bajgain, P., Sukumaran, S., Ansari, S., Heslop, H. E., Rooney, C. M., et al. (2016). Fine-tuning the CAR spacer improves T-cell potency. Oncoimmunology, 5(12), e1253656.
Feucht, J., Sun, J., Eyquem, J., Ho, Y. J., Zhao, Z., Leibold, J., et al. (2019). Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nature Medicine, 25(1), 82–88.
Sakemura, R., Terakura, S., Watanabe, K., Julamanee, J., Takagi, E., Miyao, K., et al. (2016). A Tet-On inducible system for controlling CD19-chimeric antigen receptor expression upon drug administration. Cancer Immunology Research, 4(8), 658–668.
Mestermann, K., Giavridis, T., Weber, J., Rydzek, J., Frenz, S., Nerreter, T., et al. (2019). The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Science Translational Medicine, 11(499).
Acknowledgments
The authors thank Kinderkrebshilfe Münster e.V. for the continuous support of CAR T cell research at our institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rauwolf, K.K., Rossig, C. Redirecting T cells to treat solid pediatric cancers. Cancer Metastasis Rev 38, 611–624 (2019). https://doi.org/10.1007/s10555-019-09821-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-019-09821-5