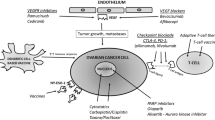
Abstract
Clinical outcomes, such as recurrence-free survival and overall survival, in ovarian cancer are quite variable, independent of common characteristics such as stage, response to therapy, and grade. This disparity in outcomes warrants further exploration and therapeutic targeting into the interaction between the tumor and host. One compelling host characteristic that contributes both to the initiation and progression of ovarian cancer is the immune system. Hundreds of studies have confirmed a prominent role for the immune system in modifying the clinical course of the disease. Recent studies also show that anti-tumor immunity is often negated by immune regulatory cells present in the tumor microenvironment. Regulatory immune cells also directly enhance the pathogenesis through the release of various cytokines and chemokines, which together form an integrated pathological network. Thus, in the future, research into immunotherapy targeting ovarian cancer will probably become increasingly focused on combination approaches that simultaneously augment immunity while preventing local immune suppression. In this article, we summarize important immunological targets that influence ovarian cancer outcome as well as include an update on newer immunotherapeutic strategies.



Similar content being viewed by others
References
Siegel, R., Naishadham, D., & Jemal, A. (2013). Cancer statistics, 2013. A Cancer Journal for Clinicians, 63(1), 11–30. doi:10.3322/caac.21166.
Aletti, G. D., Gallenberg, M. M., Cliby, W. A., Jatoi, A., & Hartmann, L. C. (2007). Current management strategies for ovarian cancer. Mayo Clinic Proceedings, 82(6), 751–770. doi:10.4065/82.6.751.
Cannistra, S. A. (2004). Cancer of the ovary. The New England Journal of Medicine, 351(24), 2519–2529. doi:10.1056/NEJMra041842.
Ozols, R. F., Bundy, B. N., Greer, B. E., Fowler, J. M., Clarke-Pearson, D., Burger, R. A., et al. (2003). Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A gynecologic oncology group study. Journal of Clinical Oncology, 21(17), 3194–3200. doi:10.1200/JCO.2003.02.153.
Vaughan, S., Coward, J. I., Bast, R. C., Jr., Berchuck, A., Berek, J. S., Brenton, J. D., et al. (2011). Rethinking ovarian cancer: Recommendations for improving outcomes. Nature Reviews Cancer, 11(10), 719–725. doi:10.1038/nrc3144.
Merritt, M. A., Green, A. C., Nagle, C. M., Webb, P. M., & S. Australian Cancer, and G. Australian Ovarian Cancer Study. (2008). Talcum powder, chronic pelvic inflammation and NSAIDs in relation to risk of epithelial ovarian cancer. International Journal of Cancer, 122(1), 170–176. doi:10.1002/ijc.23017.
Mahdavi, A., Pejovic, T., & Nezhat, F. (2006). Induction of ovulation and ovarian cancer: A critical review of the literature. Fertility and Sterility, 85(4), 819–826. doi:10.1016/j.fertnstert.2005.08.061.
Adami, H. O., Hsieh, C. C., Lambe, M., Trichopoulos, D., Leon, D., Persson, I., et al. (1994). Parity, age at first childbirth, and risk of ovarian cancer. Lancet, 344(8932), 1250–1254.
Modan, B., Hartge, P., Hirsh-Yechezkel, G., Chetrit, A., Lubin, F., Beller, U., et al. (2001). Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. The New England Journal of Medicine, 345(4), 235–240. doi:10.1056/NEJM200107263450401.
Collaborative Group on Epidemiological Studies of Ovarian, C, Beral, V., Doll, R., Hermon, C., Peto, R., & Reeves, G. (2008). Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet, 371(9609), 303–314. doi:10.1016/S0140-6736(08)60167-1.
Narod, S. A., Risch, H., Moslehi, R., Dorum, A., Neuhausen, S., Olsson, H., et al. (1998). Oral contraceptives and the risk of hereditary ovarian cancer. Hereditary ovarian cancer clinical study group. The New England Journal of Medicine, 339(7), 424–428. doi:10.1056/NEJM199808133390702.
Jordan, S. J., Cushing-Haugen, K. L., Wicklund, K. G., Doherty, J. A., & Rossing, M. A. (2012). Breast-feeding and risk of epithelial ovarian cancer. Cancer Causes & Control, 23(6), 919–927. doi:10.1007/s10552-012-9963-4.
Jordan, S. J., Siskind, V., C.Green, A., Whiteman, D. C., & Webb, P. M. (2010). Breastfeeding and risk of epithelial ovarian cancer. Cancer Causes & Control, 21(1), 109–116. doi:10.1007/s10552-009-9440-x.
Titus-Ernstoff, L., Perez, K., Cramer, D. W., Harlow, B. L., Baron, J. A., & Greenberg, E. R. (2001). Menstrual and reproductive factors in relation to ovarian cancer risk. British Journal of Cancer, 84(5), 714–721. doi:10.1054/bjoc.2000.1596.
Casagrande, J. T., Louie, E. W., Pike, M. C., Roy, S., Ross, R. K., & Henderson, B. E. (1979). “Incessant ovulation” and ovarian cancer. Lancet, 2(8135), 170–173. doi:10.1016/S0140-6736(79)91435-1.
King, S. M., Hilliard, T. S., Wu, L. Y., Jaffe, R. C., Fazleabas, A. T., & Burdette, J. E. (2011). The impact of ovulation on fallopian tube epithelial cells: Evaluating three hypotheses connecting ovulation and serous ovarian cancer. Endocrine Related Cancer, 18(5), 627–642. doi:10.1530/ERC-11-0107.
Wehner, A. P. (1994). Biological effects of cosmetic talc. Food and Chemical Toxicology, 32(12), 1173–1184.
Mills, P. K., Riordan, D. G., Cress, R. D., & Young, H. A. (2004). Perineal talc exposure and epithelial ovarian cancer risk in the Central Valley of California. International Journal of Cancer, 112(3), 458–464. doi:10.1002/ijc.20434.
Rosenblatt, K. A., Weiss, N. S., Cushing-Haugen, K. L., Wicklund, K. G., & Rossing, M. A. (2011). Genital powder exposure and the risk of epithelial ovarian cancer. Cancer Causes & Control, 22(5), 737–742. doi:10.1007/s10552-011-9746-3.
Wu, A. H., Pearce, C. L., Tseng, C. C., Templeman, C., & Pike, M. C. (2009). Markers of inflammation and risk of ovarian cancer in Los Angeles County. International Journal of Cancer, 124(6), 1409–1415. doi:10.1002/ijc.24091.
Huncharek, M., Geschwind, J. F., & Kupelnick, B. (2003). Perineal application of cosmetic talc and risk of invasive epithelial ovarian cancer: A meta-analysis of 11,933 subjects from sixteen observational studies. Anticancer Research, 23(2C), 1955–1960.
Huncharek, M., & Muscat, J. (2011). Perineal talc use and ovarian cancer risk: A case study of scientific standards in environmental epidemiology. European Journal of Cancer Prevention, 20(6), 501–507. doi:10.1097/CEJ.0b013e3283476242.
Pearce, C. L., Templeman, C., Rossing, M. A., Lee, A., Near, A. M., Webb, P. M., et al. (2012). Association between endometriosis and risk of histological subtypes of ovarian cancer: A pooled analysis of case–control studies. The Lancet Oncology, 13(4), 385–394. doi:10.1016/S1470-2045(11)70404-1.
D’Hooghe, T. M., & Debrock, S. (2002). Endometriosis, retrograde menstruation and peritoneal inflammation in women and in baboons. Human Reproduction Update, 8(1), 84–88.
Risch, H. A., & Howe, G. R. (1995). Pelvic inflammatory disease and the risk of epithelial ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention, 4(5), 447–451.
Lin, H. W., Tu, Y. Y., Lin, S. Y., Su, W. J., Lin, W. L., Lin, W. Z., et al. (2011). Risk of ovarian cancer in women with pelvic inflammatory disease: A population-based study. The Lancet Oncology, 12(9), 900–904. doi:10.1016/S1470-2045(11)70165-6.
Bonovas, S., Filioussi, K., & Sitaras, N. M. (2005). Do nonsteroidal anti-inflammatory drugs affect the risk of developing ovarian cancer? A meta-analysis. British Journal of Clinical Pharmacology, 60(2), 194–203. doi:10.1111/j.1365-2125.2005.02386.x.
Wang, Z., & Moult, J. (2001). SNPs, protein structure, and disease. Human Mutation, 17(4), 263–270. doi:10.1002/humu.22.
Bolton, K. L., Tyrer, J., Song, H., Ramus, S. J., Notaridou, M., Jones, C., et al. (2010). Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nature Genetics, 42(10), 880–884. doi:10.1038/ng.666.
Goode, E. L., Chenevix-Trench, G., Song, H., Ramus, S. J., Notaridou, M., Lawrenson, K., et al. (2010). A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nature Genetics, 42(10), 874–879. doi:10.1038/ng.668.
Song, H., Ramus, S. J., Tyrer, J., Bolton, K. L., Gentry-Maharaj, A., Wozniak, E., et al. (2009). A genome-wide association study identifies a new ovarian cancer susceptibility locus on 9p22.2. Nature Genetics, 41(9), 996–1000. doi:10.1038/ng.424.
Fan, Y., Yu, W., Ye, P., Wang, H., Wang, Z., Meng, Q., et al. (2011). NFKB1 insertion/deletion promoter polymorphism increases the risk of advanced ovarian cancer in a Chinese population. DNA and Cell Biology, 30(4), 241–245. doi:10.1089/dna.20doi:10.1107.
Lubinski, J., Huzarski, T., Kurzawski, G., Suchy, J., Masojc, B., Mierzejewski, M., et al. (2005). The 3020insC allele of NOD2 predisposes to cancers of multiple organs. Hereditary Cancer in Clinical Practice, 3(2), 59–63. doi:10.1186/1897-4287-3-2-59.
White, K. L., Vierkant, R. A., Phelan, C. M., Fridley, B. L., Anderson, S., Knutson, K. L., et al. (2009). Polymorphisms in NF-kappaB inhibitors and risk of epithelial ovarian cancer. BMC Cancer, 9, 170. doi:10.1186/1471-2407-9-170.
Ma, X., Zhang, J., Liu, S., Huang, Y., Chen, B., & Wang, D. (2011). Polymorphisms in the CASP8 gene and the risk of epithelial ovarian cancer. Gynecologic Oncology, 122(3), 554–559. doi:10.1016/j.ygyno.2011.05.031.
Engel, C., Versmold, B., Wappenschmidt, B., Simard, J., Easton, D. F., Peock, S., et al. (2010). Association of the variants CASP8 D302H and CASP10 V410I with breast and ovarian cancer risk in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiology, Biomarkers & Prevention, 19(11), 2859–2868. doi:10.1158/1055-9965.EPI-10-0517.
Nevadunsky, N. S., Korneeva, I., Caputo, T., & Witkin, S. S. (2012). Mannose-binding lectin codon 54 genetic polymorphism and vaginal protein levels in women with gynecologic malignancies. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 163(2), 216–218. doi:10.1016/j.ejogrb.2012.04.020.
Lurie, G., Terry, K. L., Wilkens, L. R., Thompson, P. J., McDuffie, K. E., Carney, M. E., et al. (2010). Pooled analysis of the association of PTGS2 rs5275 polymorphism and NSAID use with invasive ovarian carcinoma risk. Cancer Causes & Control, 21(10), 1731–1741. doi:10.1007/s10552-010-9602-x.
White, K. L., Schildkraut, J. M., Palmieri, R. T., Iversen, E. S., Jr., Berchuck, A., Vierkant, R. A., et al. (2012). Ovarian cancer risk associated with inherited inflammation-related variants. Cancer Research, 72(5), 1064–1069. doi:10.1158/0008-5472.CAN-11-3512.
Liang, D., Meyer, L., Chang, D. W., Lin, J., Pu, X., Ye, Y., et al. (2010). Genetic variants in microRNA biosynthesis pathways and binding sites modify ovarian cancer risk, survival, and treatment response. Cancer Research, 70(23), 9765–9776. doi:10.1158/0008-5472.CAN-10-0130.
Charbonneau, B., Block, M. S., Bamlet, W. R., Vierkant, R. A., Kalli, K. R., Fogarty, Z., et al. (2014). Risk of ovarian cancer and the NF-kappaB pathway: Genetic association with IL1A and TNFSF. Cancer Research, 74(3), 852–861. doi:10.1158/0008-5472.CAN-13-1051.
Palmieri, R. T., Wilson, M. A., Iversen, E. S., Clyde, M. A., Calingaert, B., Moorman, P. G., et al. (2008). Polymorphism in the IL18 gene and epithelial ovarian cancer in non-Hispanic white women. Cancer Epidemiology, Biomarkers & Prevention, 17(12), 3567–3572. doi:10.1158/1055-9965.EPI-08-0548.
Gabay, C., & Kushner, I. (1999). Acute-phase proteins and other systemic responses to inflammation. The New England Journal of Medicine, 340(6), 448–454. doi:10.1056/NEJM199902113400607.
Ridker, P. M. (2007). Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: Implications for longevity. Nutrition Reviews, 65(12 Pt 2), S253–S259.
Toriola, A. T., Grankvist, K., Agborsangaya, C. B., Lukanova, A., Lehtinen, M., & Surcel, H. M. (2011). Changes in pre-diagnostic serum C-reactive protein concentrations and ovarian cancer risk: A longitudinal study. Annals of Oncology, 22(8), 1916–1921. doi:10.1093/annonc/mdq694.
Lundin, E., Dossus, L., Clendenen, T., Krogh, V., Grankvist, K., Wulff, M., et al. (2009). C-reactive protein and ovarian cancer: A prospective study nested in three cohorts (Sweden, USA, Italy). Cancer Causes & Control, 20(7), 1151–1159. doi:10.1007/s10552-009-9330-2.
Clendenen, T. V., Lundin, E., Zeleniuch-Jacquotte, A., Koenig, K. L., Berrino, F., Lukanova, A., et al. (2011). Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention, 20(5), 799–810. doi:10.1158/1055-9965.EPI-10-1180.
Grivennikov, S. I., Greten, F. R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140(6), 883–899. doi:10.1016/j.cell.20doi:10.01.025.
Haskill, S., Becker, S., Fowler, W., & Walton, L. (1982). Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. British Journal of Cancer, 45(5), 728–736.
Zhang, L., Conejo-Garcia, J. R., Katsaros, D., Gimotty, P. A., Massobrio, M., Regnani, G., et al. (2003). Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. The New England Journal of Medicine, 348(3), 203–213. doi:10.1056/NEJMoa020177.
Sato, E., Olson, S. H., Ahn, J., Bundy, B., Nishikawa, H., Qian, F., et al. (2005). Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 102(51), 18538–18543. doi:10.1073/pnas.0509182102.
Leffers, N., Gooden, M. J., de Jong, R. A., Hoogeboom, B. N., ten Hoor, K. A., Hollema, H., et al. (2009). Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunology, Immunotherapy, 58(3), 449–459. doi:10.1007/s00262-008-0583-5.
Callahan, M. J., Nagymanyoki, Z., Bonome, T., Johnson, M. E., Litkouhi, B., Sullivan, E. H., et al. (2008). Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clinical Cancer Research, 14(23), 7667–7673. doi:10.1158/1078-0432.CCR-08-0479.
Leffers, N., Fehrmann, R. S., Gooden, M. J., Schulze, U. R., Ten Hoor, K. A., Hollema, H., et al. (2010). Identification of genes and pathways associated with cytotoxic T lymphocyte infiltration of serous ovarian cancer. British Journal of Cancer, 103(5), 685–692. doi:10.1038/sj.bjc.6605820.
Milne, K., Kobel, M., Kalloger, S. E., Barnes, R. O., Gao, D., Gilks, C. B., et al. (2009). Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PloS One, 4(7), e6412. doi:10.1371/journal.pone.0006412.
Le Page, C., Marineau, A., Bonza, P. K., Rahimi, K., Cyr, L., Labouba, I., et al. (2012). BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PloS One, 7(6), e38541. doi:10.1371/journal.pone.0038541.
Kryczek, I., Banerjee, M., Cheng, P., Vatan, L., Szeliga, W., Wei, S., et al. (2009). Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood, 114(6), 1141–1149. doi:10.1182/blood-2009-03-208249.
Cua, D. J., & Tato, C. M. (2010). Innate IL-17-producing cells: The sentinels of the immune system. Nature Reviews Immunology, 10(7), 479–489. doi:10.1038/nri2800.
Goodell, V., Salazar, L. G., Urban, N., Drescher, C. W., Gray, H., Swensen, R. E., et al. (2006). Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. Journal of Clinical Oncology, 24(5), 762–768. doi:10.1200/JCO.2005.03.2813.
Knutson, K. L., Krco, C. J., Erskine, C. L., Goodman, K., Kelemen, L. E., Wettstein, P. J., et al. (2006). T-cell immunity to the folate receptor alpha is prevalent in women with breast or ovarian cancer. Journal of Clinical Oncology, 24(26), 4254–4261. doi:10.1200/JCO.2006.05.9311.
Tchabo, N. E., Mhawech-Fauceglia, P., Caballero, O. L., Villella, J., Beck, A. F., Miliotto, A. J., et al. (2009). Expression and serum immunoreactivity of developmentally restricted differentiation antigens in epithelial ovarian cancer. Cancer Immunity, 9, 6.
Dong, H. P., Elstrand, M. B., Holth, A., Silins, I., Berner, A., Trope, C. G., et al. (2006). NK- and B-cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. American Journal of Clinical Pathology, 125(3), 451–458.
Nielsen, J. S., Sahota, R. A., Milne, K., Kost, S. E., Nesslinger, N. J., Watson, P. H., et al. (2012). CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clinical Cancer Research, 18(12), 3281–3292. doi:10.1158/1078-0432.CCR-12-0234.
Perussia, B., Chen, Y., & Loza, M. J. (2005). Peripheral NK cell phenotypes: Multiple changing of faces of an adapting, developing cell. Molecular Immunology, 42(4), 385–395. doi:10.1016/j.molimm.2004.07.017.
Gonzalez, S., Lopez-Soto, A., Suarez-Alvarez, B., Lopez-Vazquez, A., & Lopez-Larrea, C. (2008). NKG2D ligands: Key targets of the immune response. Trends in Immunology, 29(8), 397–403. doi:10.1016/j.it.2008.04.007.
Lopez-Larrea, C., Suarez-Alvarez, B., Lopez-Soto, A., Lopez-Vazquez, A., & Gonzalez, S. (2008). The NKG2D receptor: Sensing stressed cells. Trends in Molecular Medicine, 14(4), 179–189. doi:10.1016/j.molmed.2008.02.004.
Li, K., Mandai, M., Hamanishi, J., Matsumura, N., Suzuki, A., Yagi, H., et al. (2009). Clinical significance of the NKG2D ligands, MICA/B and ULBP2 in ovarian cancer: High expression of ULBP2 is an indicator of poor prognosis. Cancer Immunology, Immunotherapy, 58(5), 641–652. doi:10.1007/s00262-008-0585-3.
Knutson, K. L., Disis, M. L., & Salazar, L. G. (2007). CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunology, Immunotherapy, 56(3), 271–285. doi:10.1007/s00262-006-0194-y.
Bluestone, J. A., & Abbas, A. K. (2003). Natural versus adaptive regulatory T cells. Nature Reviews Immunology, 3(3), 253–257. doi:10.1038/nri1032.
Meloni, F., Morosini, M., Solari, N., Passadore, I., Nascimbene, C., Novo, M., et al. (2006). Foxp3 expressing CD4+ CD25+ and CD8+ CD28− T regulatory cells in the peripheral blood of patients with lung cancer and pleural mesothelioma. Human Immunology, 67(1–2), 1–12. doi:10.1016/j.humimm.2005.11.005.
Audia, S., Nicolas, A., Cathelin, D., Larmonier, N., Ferrand, C., Foucher, P., et al. (2007). Increase of CD4+ CD25+ regulatory T cells in the peripheral blood of patients with metastatic carcinoma: A phase I clinical trial using cyclophosphamide and immunotherapy to eliminate CD4+ CD25+ T lymphocytes. Clinical and Experimental Immunology, 150(3), 523–530. doi:10.1111/j.1365-2249.2007.03521.x.
Li, X., Ye, D. F., Xie, X., Chen, H. Z., & Lu, W. G. (2005). Proportion of CD4+ CD25+ regulatory T cell is increased in the patients with ovarian carcinoma. Cancer Investigation, 23(5), 399–403.
Preston, C. C., Maurer, M. J., Oberg, A. L., Visscher, D. W., Kalli, K. R., Hartmann, L. C., et al. (2013). The ratios of CD8+ T cells to CD4+ CD25+ FOXP3+ and FOXP3− T cells correlate with poor clinical outcome in human serous ovarian cancer. PloS One, 8(11), e80063. doi:10.1371/journal.pone.0080063.
Curiel, T. J., Coukos, G., Zou, L., Alvarez, X., Cheng, P., Mottram, P., et al. (2004). Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine, 10(9), 942–949. doi:10.1038/nm1093.
Wolf, D., Wolf, A. M., Rumpold, H., Fiegl, H., Zeimet, A. G., Muller-Holzner, E., et al. (2005). The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clinical Cancer Research, 11(23), 8326–8331. doi:10.1158/1078-0432.CCR-05-1244.
Murray, P. J., & Wynn, T. A. (2011). Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology, 11(11), 723–737. doi:10.1038/nri3073.
Hagemann, T., Wilson, J., Burke, F., Kulbe, H., Li, N. F., Pluddemann, A., et al. (2006). Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. Journal of Immunology, 176(8), 5023–5032.
Ko, S. Y., Ladanyi, A., Lengyel, E., & Naora, H. (2014). Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype. The American Journal of Pathology, 184(1), 271–281. doi:10.1016/j.ajpath.2013.09.017.
Shah, C. A., Allison, K. H., Garcia, R. L., Gray, H. J., Goff, B. A., & Swisher, E. M. (2008). Intratumoral T cells, tumor-associated macrophages, and regulatory T cells: Association with p53 mutations, circulating tumor DNA and survival in women with ovarian cancer. Gynecologic Oncology, 109(2), 215–219. doi:10.1016/j.ygyno.2008.01.0doi:10.
Lan, C., Huang, X., Lin, S., Huang, H., Cai, Q., Wan, T., et al. (2013). Expression of M2-polarized macrophages is associated with poor prognosis for advanced epithelial ovarian cancer. Technology in Cancer Research & Treatment, 12(3), 259–267. doi:10.7785/tcrt.2012.500312.
Karyampudi, L., Lamichhane, P., Scheid, A. D., Kalli, K. R., Shreeder, B., Krempski, J. W., et al. (2014). Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Research, 74(11), 2974–2985. doi:10.1158/0008-5472.CAN-13-2564.
Fricke, I., & Gabrilovich, D. I. (2006). Dendritic cells and tumor microenvironment: A dangerous liaison. Immunological Investigations, 35(3–4), 459–483. doi:10.1080/08820130600803429.
Chen, F., Hou, M., Ye, F., Lv, W., & Xie, X. (2009). Ovarian cancer cells induce peripheral mature dendritic cells to differentiate into macrophagelike cells in vitro. International Journal of Gynecological Cancer, 19(9), 1487–1493. doi:10.1111/IGC.0b013e3181bb70c6.
Krempski, J., Karyampudi, L., Behrens, M. D., Erskine, C. L., Hartmann, L., Dong, H., et al. (2011). Tumor-infiltrating programmed death receptor-1+ dendritic cells mediate immune suppression in ovarian cancer. Journal of Immunology, 186(12), 6905–6913. doi:10.4049/jimmunol.1100274.
Schmid, M. C., & Varner, J. A. (2010). Myeloid cells in the tumor microenvironment: Modulation of tumor angiogenesis and tumor inflammation. Journal of Oncology, 2010, 201026. doi:10.1155/2010/201026.
Ostrand-Rosenberg, S. (2010). Myeloid-derived suppressor cells: More mechanisms for inhibiting antitumor immunity. Cancer Immunology, Immunotherapy, 59(10), 1593–1600. doi:10.1007/s00262-010-0855-8.
Kerbel, R. S. (2008). Tumor angiogenesis. The New England Journal of Medicine, 358(19), 2039–2049. doi:10.1056/NEJMra0706596.
Zou, W., Machelon, V., Coulomb-L’Hermin, A., Borvak, J., Nome, F., Isaeva, T., et al. (2001). Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nature Medicine, 7(12), 1339–1346. doi:10.1038/nm1201-1339.
Wei, S., Kryczek, I., Zou, L., Daniel, B., Cheng, P., Mottram, P., et al. (2005). Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Research, 65(12), 5020–5026. doi:10.1158/0008-5472.CAN-04-4043.
Huarte, E., Cubillos-Ruiz, J. R., Nesbeth, Y. C., Scarlett, U. K., Martinez, D. G., Buckanovich, R. J., et al. (2008). Depletion of dendritic cells delays ovarian cancer progression by boosting antitumor immunity. Cancer Research, 68(18), 7684–7691. doi:10.1158/0008-5472.CAN-08-1167.
Gabrilovich, D. I., & Nagaraj, S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology, 9(3), 162–174. doi:10.1038/nri2506.
Movahedi, K., Guilliams, M., Van den Bossche, J., Van den Bergh, R., Gysemans, C., Beschin, A., et al. (2008). Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood, 111(8), 4233–4244. doi:10.1182/blood-2007-07-099226.
Youn, J. I., Nagaraj, S., Collazo, M., & Gabrilovich, D. I. (2008). Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of Immunology, 181(8), 5791–5802.
Serafini, P., Borrello, I., & Bronte, V. (2006). Myeloid suppressor cells in cancer: Recruitment, phenotype, properties, and mechanisms of immune suppression. Seminars in Cancer Biology, 16(1), 53–65. doi:10.1016/j.semcancer.2005.07.005.
Yang, R., Cai, Z., Zhang, Y., Yutzy, W. H., Roby, K. F., & Roden, R. B. (2006). CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+ CD11b+ myeloid cells. Cancer Research, 66(13), 6807–6815. doi:10.1158/0008-5472.CAN-05-3755.
Hamanishi, J., Mandai, M., Iwasaki, M., Okazaki, T., Tanaka, Y., Yamaguchi, K., et al. (2007). Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 104(9), 3360–3365. doi:10.1073/pnas.0611533104.
Ghebeh, H., Mohammed, S., Al-Omair, A., Qattan, A., Lehe, C., Al-Qudaihi, G., et al. (2006). The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: Correlation with important high-risk prognostic factors. Neoplasia, 8(3), 190–198. doi:10.1593/neo.05733.
Karim, R., Jordanova, E. S., Piersma, S. J., Kenter, G. G., Chen, L., Boer, J. M., et al. (2009). Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clinical Cancer Research, 15(20), 6341–6347. doi:10.1158/1078-0432.CCR-09-1652.
Keir, M. E., Butte, M. J., Freeman, G. J., & Sharpe, A. H. (2008). PD-1 and its ligands in tolerance and immunity. Annual Review of Immunology, 26, 677–704. doi:10.1146/annurev.immunol.26.021607.090331.
Keir, M. E., Francisco, L. M., & Sharpe, A. H. (2007). PD-1 and its ligands in T-cell immunity. Current Opinion in Immunology, 19(3), 309–314. doi:10.1016/j.coi.2007.04.012.
Keir, M. E., Liang, S. C., Guleria, I., Latchman, Y. E., Qipo, A., Albacker, L. A., et al. (2006). Tissue expression of PD-L1 mediates peripheral T cell tolerance. The Journal of Experimental Medicine, 203(4), 883–895. doi:10.1084/jem.20051776.
Matsuzaki, J., Gnjatic, S., Mhawech-Fauceglia, P., Beck, A., Miller, A., Tsuji, T., et al. (2010). Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America, 107(17), 7875–7880. doi:10.1073/pnas.1003345107.
Gubbels, J. A., Belisle, J., Onda, M., Rancourt, C., Migneault, M., Ho, M., et al. (2006). Mesothelin-MUC16 binding is a high affinity, N-glycan dependent interaction that facilitates peritoneal metastasis of ovarian tumors. Molecular Cancer, 5(1), 50. doi:10.1186/1476-4598-5-50.
Patankar, M. S., Jing, Y., Morrison, J. C., Belisle, J. A., Lattanzio, F. A., Deng, Y., et al. (2005). Potent suppression of natural killer cell response mediated by the ovarian tumor marker CA125. Gynecologic Oncology, 99(3), 704–713. doi:10.1016/j.ygyno.2005.07.030.
Gubbels, J. A., Felder, M., Horibata, S., Belisle, J. A., Kapur, A., Holden, H., et al. (2010). MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Molecular Cancer, 9, 11. doi:10.1186/1476-4598-9-11.
Belisle, J. A., Horibata, S., Jennifer, G. A., Petrie, S., Kapur, A., Andre, S., et al. (2010). Identification of siglec-9 as the receptor for MUC16 on human NK cells, B cells, and monocytes. Molecular Cancer, 9, 118. doi:10.1186/1476-4598-9-118.
Krockenberger, M., Dombrowski, Y., Weidler, C., Ossadnik, M., Honig, A., Hausler, S., et al. (2008). Macrophage migration inhibitory factor contributes to the immune escape of ovarian cancer by down-regulating NKG2D. Journal of Immunology, 180(11), 7338–7348.
Uyttenhove, C., Pilotte, L., Theate, I., Stroobant, V., Colau, D., Parmentier, N., et al. (2003). Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nature Medicine, 9(10), 1269–1274. doi:10.1038/nm934.
Munn, D. H. (2006). Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Current Opinion in Immunology, 18(2), 220–225. doi:10.1016/j.coi.2006.01.002.
Terness, P., Bauer, T. M., Rose, L., Dufter, C., Watzlik, A., Simon, H., et al. (2002). Inhibition of allogeneic T cell proliferation by indoleamine 2,3-dioxygenase-expressing dendritic cells: Mediation of suppression by tryptophan metabolites. The Journal of Experimental Medicine, 196(4), 447–457.
Munn, D. H., Sharma, M. D., Baban, B., Harding, H. P., Zhang, Y., Ron, D., et al. (2005). GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity, 22(5), 633–642. doi:10.1016/j.immuni.2005.03.013.
Della Chiesa, M., Carlomagno, S., Frumento, G., Balsamo, M., Cantoni, C., Conte, R., et al. (2006). The tryptophan catabolite L-kynurenine inhibits the surface expression of NKp46- and NKG2D-activating receptors and regulates NK-cell function. Blood, 108(13), 4118–4125. doi:10.1182/blood-2006-03-006700.
Chiesa, S., Mingueneau, M., Fuseri, N., Malissen, B., Raulet, D. H., Malissen, M., et al. (2006). Multiplicity and plasticity of natural killer cell signaling pathways. Blood, 107(6), 2364–2372. doi:10.1182/blood-2005-08-3504.
Frumento, G., Rotondo, R., Tonetti, M., Damonte, G., Benatti, U., & Ferrara, G. B. (2002). Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. The Journal of Experimental Medicine, 196(4), 459–468.
Baban, B., Chandler, P. R., Sharma, M. D., Pihkala, J., Koni, P. A., Munn, D. H., et al. (2009). IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. Journal of Immunology, 183(4), 2475–2483. doi:10.4049/jimmunol.0900986.
Fallarino, F., Grohmann, U., You, S., McGrath, B. C., Cavener, D. R., Vacca, C., et al. (2006). The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. Journal of Immunology, 176(11), 6752–6761.
Hill, M., Tanguy-Royer, S., Royer, P., Chauveau, C., Asghar, K., Tesson, L., et al. (2007). IDO expands human CD4+ CD25 high regulatory T cells by promoting maturation of LPS-treated dendritic cells. European Journal of Immunology, 37(11), 3054–3062. doi:10.1002/eji.200636704.
Inaba, T., Ino, K., Kajiyama, H., Yamamoto, E., Shibata, K., Nawa, A., et al. (2009). Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecologic Oncology, 115(2), 185–192. doi:10.1016/j.ygyno.2009.07.015.
Okamoto, A., Nikaido, T., Ochiai, K., Takakura, S., Saito, M., Aoki, Y., et al. (2005). Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clinical Cancer Research, 11(16), 6030–6039. doi:10.1158/1078-0432.CCR-04-2671.
Takao, M., Okamoto, A., Nikaido, T., Urashima, M., Takakura, S., Saito, M., et al. (2007). Increased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancer. Oncology Reports, 17(6), 1333–1339.
Zheng, X., Koropatnick, J., Li, M., Zhang, X., Ling, F., Ren, X., et al. (2006). Reinstalling antitumor immunity by inhibiting tumor-derived immunosuppressive molecule IDO through RNA interference. Journal of Immunology, 177(8), 5639–5646.
Zamanakou, M., Germenis, A. E., & Karanikas, V. (2007). Tumor immune escape mediated by indoleamine 2,3-dioxygenase. Immunology Letters, 111(2), 69–75. doi:10.1016/j.imlet.2007.06.001.
Lob, S., Konigsrainer, A., Zieker, D., Brucher, B. L., Rammensee, H. G., Opelz, G., et al. (2009). IDO1 and IDO2 are expressed in human tumors: Levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer Immunology, Immunotherapy, 58(1), 153–157. doi:10.1007/s00262-008-0513-6.
Liu, X., Shin, N., Koblish, H. K., Yang, G., Wang, Q., Wang, K., et al. (2010). Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood, 115(17), 3520–3530. doi:10.1182/blood-2009-09-246124.
Koblish, H. K., Hansbury, M. J., Bowman, K. J., Yang, G., Neilan, C. L., Haley, P. J., et al. (2010). Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Molecular Cancer Therapeutics, 9(2), 489–498. doi:10.1158/1535-7163.MCT-09-0628.
Soliman, H., Mediavilla-Varela, M., & Antonia, S. (2010). Indoleamine 2,3-dioxygenase: Is it an immune suppressor? Cancer Journal, 16(4), 354–359.
Cady, S. G., & Sono, M. (1991). 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Archives of Biochemistry and Biophysics, 291(2), 326–333.
Hou, D. Y., Muller, A. J., Sharma, M. D., DuHadaway, J., Banerjee, T., Johnson, M., et al. (2007). Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Research, 67(2), 792–801. doi:10.1158/0008-5472.CAN-06-2925.
Metz, R., Duhadaway, J. B., Kamasani, U., Laury-Kleintop, L., Muller, A. J., & Prendergast, G. C. (2007). Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Research, 67(15), 7082–7087. doi:10.1158/0008-5472.CAN-07-1872.
Opitz, C. A., Litzenburger, U. M., Sahm, F., Ott, M., Tritschler, I., Trump, S., et al. (2011). An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature, 478(7368), 197–203. doi:10.1038/nature10491.
Quintana, F. J., Basso, A. S., Iglesias, A. H., Korn, T., Farez, M. F., Bettelli, E., et al. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature, 453(7191), 65–71. doi:10.1038/nature06880.
Pilotte, L., Larrieu, P., Stroobant, V., Colau, D., Dolusic, E., Frederick, R., et al. (2012). Reversal of tumoral immune resistance by inhibition of tryptophan 2,3-dioxygenase. Proceedings of the National Academy of Sciences of the United States of America, 109(7), 2497–2502. doi:10.1073/pnas.1113873109.
Kulbe, H., Chakravarty, P., Leinster, D. A., Charles, K. A., Kwong, J., Thompson, R. G., et al. (2012). A dynamic inflammatory cytokine network in the human ovarian cancer microenvironment. Cancer Research, 72(1), 66–75. doi:10.1158/0008-5472.CAN-11-2178.
Kulbe, H., Thompson, R., Wilson, J. L., Robinson, S., Hagemann, T., Fatah, R., et al. (2007). The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells. Cancer Research, 67(2), 585–592. doi:10.1158/0008-5472.CAN-06-2941.
Balkwill, F. (2002). Tumor necrosis factor or tumor promoting factor? Cytokine & Growth Factor Reviews, 13(2), 135–141. doi:10.1016/s1359-6101(01)00020-x.
Anderson, G. M., Nakada, M. T., & DeWitte, M. (2004). Tumor necrosis factor-alpha in the pathogenesis and treatment of cancer. Current Opinion in Pharmacology, 4(4), 314–320. doi:10.1016/j.coph.2004.04.004.
Penson, R. T., Kronish, K., Duan, Z., Feller, A. J., Stark, P., Cook, S. E., et al. (2000). Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. International Journal of Gynecological Cancer, 10(1), 33–41. doi:10.1046/j.1525-1438.2000.00003.x.
Guo, Y., Xu, F., Lu, T., Duan, Z., & Zhang, Z. (2012). Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treatment Reviews, 38(7), 904–909. doi:10.1016/j.ctrv.2012.04.007.
Coward, J., Kulbe, H., Chakravarty, P., Leader, D., Vassileva, V., Leinster, D. A., et al. (2011). Interleukin-6 as a therapeutic target in human ovarian cancer. Clinical Cancer Research, 17(18), 6083–6096. doi:10.1158/1078-0432.CCR-11-0945.
Duan, Z., Foster, R., Bell, D. A., Mahoney, J., Wolak, K., Vaidya, A., et al. (2006). Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clinical Cancer Research, 12(17), 5055–5063. doi:10.1158/1078-0432.CCR-06-0861.
Lo, C. W., Chen, M. W., Hsiao, M., Wang, S., Chen, C. A., Hsiao, S. M., et al. (2011). IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Research, 71(2), 424–434. doi:10.1158/0008-5472.CAN-10-1496.
Plante, M., Rubin, S. C., Wong, G. Y., Federici, M. G., Finstad, C. L., & Gastl, G. A. (1994). Interleukin-6 level in serum and ascites as a prognostic factor in patients with epithelial ovarian cancer. Cancer, 73(7), 1882–1888. doi:10.1002/1097-0142(19940401)73:7<1882::aid-cncr2820730718>3.0.co;2-r.
Yang, L., Wang, L., Lin, H. K., Kan, P. Y., Xie, S., Tsai, M. Y., et al. (2003). Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochemical and Biophysical Research Communications, 305(3), 462–469. doi:10.1016/s0006-291x(03)00792-7.
Wang, T. H., Chan, Y. H., Chen, C. W., Kung, W. H., Lee, Y. S., Wang, S. T., et al. (2006). Paclitaxel (Taxol) upregulates expression of functional interleukin-6 in human ovarian cancer cells through multiple signaling pathways. Oncogene, 25(35), 4857–4866.
Stone, R. L., Nick, A. M., McNeish, I. A., Balkwill, F., Han, H. D., Bottsford-Miller, J., et al. (2012). Paraneoplastic thrombocytosis in ovarian cancer. The New England Journal of Medicine, 366(7), 610–618. doi:10.1056/NEJMoa1110352.
Trikha, M., Corringham, R., Klein, B., & Rossi, J. F. (2003). Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: A review of the rationale and clinical evidence. Clinical Cancer Research, 9(13), 4653–4665.
Barbieri, F., Bajetto, A., & Florio, T. (2010). Role of chemokine network in the development and progression of ovarian cancer: a potential novel pharmacological target. Journal of Oncology, 2010, 426956. doi:10.1155/2010/426956.
Kryczek, I., Lange, A., Mottram, P., Alvarez, X., Cheng, P., Hogan, M., et al. (2005). CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Research, 65(2), 465–472.
Porcile, C., Bajetto, A., Barbieri, F., Barbero, S., Bonavia, R., Biglieri, M., et al. (2005). Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Experimental Cell Research, 308(2), 241–253. doi:10.1016/j.yexcr.2005.04.024.
Kajiyama, H., Shibata, K., Terauchi, M., Ino, K., Nawa, A., & Kikkawa, F. (2008). Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma. International Journal of Cancer, 122(1), 91–99. doi:10.1002/ijc.23083.
Righi, E., Kashiwagi, S., Yuan, J., Santosuosso, M., Leblanc, P., Ingraham, R., et al. (2011). CXCL12/CXCR4 blockade induces multimodal antitumor effects that prolong survival in an immunocompetent mouse model of ovarian cancer. Cancer Research, 71(16), 5522–5534. doi:10.1158/0008-5472.CAN-10-3143.
Goode, E. L., Maurer, M. J., Sellers, T. A., Phelan, C. M., Kalli, K. R., Fridley, B. L., et al. (2010). Inherited determinants of ovarian cancer survival. Clinical Cancer Research, 16(3), 995–1007. doi:10.1158/1078-0432.CCR-09-2553.
Frede, S., Freitag, P., Otto, T., Heilmaier, C., & Fandrey, J. (2005). The proinflammatory cytokine interleukin 1beta and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Research, 65(11), 4690–4697. doi:10.1158/0008-5472.CAN-04-3877.
Ioana Braicu, E., Mustea, A., Toliat, M. R., Pirvulescu, C., Konsgen, D., Sun, P., et al. (2007). Polymorphism of IL-1alpha, IL-1beta and IL-10 in patients with advanced ovarian cancer: Results of a prospective study with 147 patients. Gynecologic Oncology, 104(3), 680–685. doi:10.1016/j.ygyno.2006.doi:10.014.
Goode, E. L., DeRycke, M., Kalli, K. R., Oberg, A. L., Cunningham, J. M., Maurer, M. J., et al. (2013). Inherited variants in regulatory T cell genes and outcome of ovarian cancer. PloS One, 8(1), e53903. doi:10.1371/journal.pone.0053903.
Charbonneau, B., Moysich, K. B., Kalli, K. R., Oberg, A. L., Vierkant, R. A., Fogarty, Z. C., et al. (2014). Large-scale evaluation of common variation in regulatory T cell-related genes and ovarian cancer outcome. Cancer Immunology Research, 2(4), 332–340. doi:10.1158/2326-6066.CIR-13-0136.
Ayyoub, M., Pignon, P., Classe, J. M., Odunsi, K., & Valmori, D. (2013). CD4+ T effectors specific for the tumor antigen NY-ESO-1 are highly enriched at ovarian cancer sites and coexist with, but are distinct from, tumor-associated treg. Cancer Immunology Research, 1(5), 303–308. doi:10.1158/2326-6066.CIR-13-0062-T.
Wick, D. A., Webb, J. R., Nielsen, J. S., Martin, S. D., Kroeger, D. R., Milne, K., et al. (2014). Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clinical Cancer Research, 20(5), 1125–1134. doi:10.1158/1078-0432.CCR-13-2147.
Ioannides, C. G., Fisk, B., Fan, D., Biddison, W. E., Wharton, J. T., & O’Brian, C. A. (1993). Cytotoxic T cells isolated from ovarian malignant ascites recognize a peptide derived from the HER-2/neu proto-oncogene. Cellular Immunology, 151(1), 225–234. doi:10.1006/cimm.1993.1233.
Doherty, J. K., Bond, C., Jardim, A., Adelman, J. P., & Clinton, G. M. (1999). The HER-2/neu receptor tyrosine kinase gene encodes a secreted autoinhibitor. Proceedings of the National Academy of Sciences of the United States of America, 96(19), 10869–10874.
Bookman, M. A., Darcy, K. M., Clarke-Pearson, D., Boothby, R. A., & Horowitz, I. R. (2003). Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: A phase II trial of the gynecologic oncology group. Journal of Clinical Oncology, 21(2), 283–290.
Camilleri-Broet, S., Hardy-Bessard, A. C., Le Tourneau, A., Paraiso, D., Levrel, O., Leduc, B., et al. (2004). HER-2 overexpression is an independent marker of poor prognosis of advanced primary ovarian carcinoma: A multicenter study of the GINECO group. Annals of Oncology, 15(1), 104–112.
Karaferic, A., Jovanovic, D., & Jelic, S. (2009). Expression of HER2/neu, estrogen and progesterone receptors, CA 125 and CA19-9 on cancer cell membrane in patients with serous and mucinous carcinoma of the ovary. Journal of B.U.ON., 14(4), 635–639.
Tuefferd, M., Couturier, J., Penault-Llorca, F., Vincent-Salomon, A., Broet, P., Guastalla, J. P., et al. (2007). HER2 status in ovarian carcinomas: A multicenter GINECO study of 320 patients. PloS One, 2(11), e1138. doi:10.1371/journal.pone.0001138.
Hogdall, E. V., Christensen, L., Kjaer, S. K., Blaakaer, J., Bock, J. E., Glud, E., et al. (2003). Distribution of HER-2 overexpression in ovarian carcinoma tissue and its prognostic value in patients with ovarian carcinoma: From the Danish MALOVA ovarian cancer study. Cancer, 98(1), 66–73. doi:10.1002/cncr.11476.
Baron-Hay, S., Boyle, F., Ferrier, A., & Scott, C. (2004). Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clinical Cancer Research, 10(5), 1796–1806.
Lancaster, J. M., Sayer, R. A., Blanchette, C., Calingaert, B., Konidari, I., Gray, J., et al. (2006). High expression of insulin-like growth factor binding protein-2 messenger RNA in epithelial ovarian cancers produces elevated preoperative serum levels. International Journal of Gynecological Cancer, 16(4), 1529–1535. doi:10.1111/j.1525-1438.2006.00623.x.
Wang, H., Rosen, D. G., Wang, H., Fuller, G. N., Zhang, W., & Liu, J. (2006). Insulin-like growth factor-binding protein 2 and 5 are differentially regulated in ovarian cancer of different histologic types. Modern Pathology, 19(9), 1149–1156. doi:10.1038/modpathol.3800637.
Yan, X. J., Tian, Y., Wang, C., Wang, X. L., Di, J. M., & Cheng, J. X. (2009). The expressions and clinical significance of IGFBP-2, -3 in both serum and tumor tissues in patients with epithelial ovarian cancer. Sichuan Da Xue Xue Bao. Yi Xue Ban, 40(4), 639–643.
Kalli, K. R., Krco, C. J., Hartmann, L. C., Goodman, K., Maurer, M. J., Yu, C., et al. (2008). An HLA-DR-degenerate epitope pool detects insulin-like growth factor binding protein 2-specific immunity in patients with cancer. Cancer Research, 68(12), 4893–4901. doi:10.1158/0008-5472.CAN-07-6726.
Bafna, S., Singh, A. P., Moniaux, N., Eudy, J. D., Meza, J. L., & Batra, S. K. (2008). MUC4, a multifunctional transmembrane glycoprotein, induces oncogenic transformation of NIH3T3 mouse fibroblast cells. Cancer Research, 68(22), 9231–9238. doi:10.1158/0008-5472.CAN-08-3135.
Senapati, S., Sharma, P., Bafna, S., Roy, H. K., & Batra, S. K. (2008). The MUC gene family: Their role in the diagnosis and prognosis of gastric cancer. Histology and Histopathology, 23(12), 1541–1552.
Chauhan, S. C., Singh, A. P., Ruiz, F., Johansson, S. L., Jain, M., Smith, L. M., et al. (2006). Aberrant expression of MUC4 in ovarian carcinoma: Diagnostic significance alone and in combination with MUC1 and MUC16 (CA125). Modern Pathology, 19(10), 1386–1394. doi:10.1038/modpathol.3800646.
Singh, A. P., Chauhan, S. C., Bafna, S., Johansson, S. L., Smith, L. M., Moniaux, N., et al. (2006). Aberrant expression of transmembrane mucins, MUC1 and MUC4, in human prostate carcinomas. The Prostate, 66(4), 421–429. doi:10.1002/pros.20372.
Bast, R. C., Jr., Xu, F. J., Yu, Y. H., Barnhill, S., Zhang, Z., & Mills, G. B. (1998). CA 125: The past and the future. The International Journal of Biological Markers, 13(4), 179–187.
Yin, B. W., & Lloyd, K. O. (2001). Molecular cloning of the CA125 ovarian cancer antigen: Identification as a new mucin, MUC16. The Journal of Biological Chemistry, 276(29), 27371–27375. doi:10.1074/jbc.M103554200.
Ponnusamy, M. P., Lakshmanan, I., Jain, M., Das, S., Chakraborty, S., Dey, P., et al. (2010). MUC4 mucin-induced epithelial to mesenchymal transition: A novel mechanism for metastasis of human ovarian cancer cells. Oncogene, 29(42), 5741–5754. doi:10.1038/onc.20doi:10.309.
Terry, K. L., Titus-Ernstoff, L., McKolanis, J. R., Welch, W. R., Finn, O. J., & Cramer, D. W. (2007). Incessant ovulation, mucin 1 immunity, and risk for ovarian cancer. Cancer Epidemiology, Biomarkers & Prevention, 16(1), 30–35. doi:10.1158/1055-9965.EPI-06-0688.
Oei, A. L., Moreno, M., Verheijen, R. H., Sweep, F. C., Thomas, C. M., Massuger, L. F., et al. (2008). Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. International Journal of Cancer, 123(8), 1848–1853. doi:10.1002/ijc.23725.
Plisiecka-Halasa, J., Dansonka-Mieszkowska, A., Kraszewska, E., Danska-Bidzinska, A., & Kupryjanczyk, J. (2008). Loss of heterozygosity, microsatellite instability and TP53 gene status in ovarian carcinomas. Anticancer Research, 28(2A), 989–996.
Corney, D. C., Flesken-Nikitin, A., Choi, J., & Nikitin, A. Y. (2008). Role of p53 and Rb in ovarian cancer. Advances in Experimental Medicine and Biology, 622, 99–117. doi:10.1007/978-0-387-68969-2_9.
Lambeck, A., Leffers, N., Hoogeboom, B. N., Sluiter, W., Hamming, I., Klip, H., et al. (2007). P53-specific T cell responses in patients with malignant and benign ovarian tumors: Implications for p53 based immunotherapy. International Journal of Cancer, 121(3), 606–614. doi:10.1002/ijc.227doi:10.
Valmori, D., Qian, F., Ayyoub, M., Renner, C., Merlo, A., Gnjatic, S., et al. (2006). Expression of synovial sarcoma X (SSX) antigens in epithelial ovarian cancer and identification of SSX-4 epitopes recognized by CD4+ T cells. Clinical Cancer Research, 12(2), 398–404. doi:10.1158/1078-0432.CCR-05-1902.
Zhang, S., Zhou, X., Yu, H., & Yu, Y. (2010). Expression of tumor-specific antigen MAGE, GAGE and BAGE in ovarian cancer tissues and cell lines. BMC Cancer, 10, 163. doi:10.1186/1471-2407-10-163.
Hofmann, M., & Ruschenburg, I. (2002). mRNA detection of tumor-rejection genes BAGE, GAGE, and MAGE in peritoneal fluid from patients with ovarian carcinoma as a potential diagnostic tool. Cancer, 96(3), 187–193. doi:10.1002/cncr.10622.
Straughn, J. M., Jr., Shaw, D. R., Guerrero, A., Bhoola, S. M., Racelis, A., Wang, Z., et al. (2004). Expression of sperm protein 17 (Sp17) in ovarian cancer. International Journal of Cancer, 108(6), 805–811. doi:10.1002/ijc.11617.
Odunsi, K., Jungbluth, A. A., Stockert, E., Qian, F., Gnjatic, S., Tammela, J., et al. (2003). NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Research, 63(18), 6076–6083.
Agarwal, S., Saini, S., Parashar, D., Verma, A., Sinha, A., Jagadish, N., et al. (2013). The novel cancer-testis antigen A-kinase anchor protein 4 (AKAP4) is a potential target for immunotherapy of ovarian serous carcinoma. Oncoimmunology, 2(5), e24270. doi:10.4161/onci.24270.
Li, F. Q., Han, Y. L., Liu, Q., Wu, B., Huang, W. B., & Zeng, S. Y. (2009). Overexpression of human sperm protein 17 increases migration and decreases the chemosensitivity of human epithelial ovarian cancer cells. BMC Cancer, 9, 323. doi:10.1186/1471-2407-9-323.
Brown Jones, M., Neuper, C., Clayton, A., Mariani, A., Konecny, G., Thomas, M. B., et al. (2008). Rationale for folate receptor alpha targeted therapy in “high risk” endometrial carcinomas. International Journal of Cancer, 123(7), 1699–1703.
Dainty, L. A., Risinger, J. I., Morrison, C., Chandramouli, G. V., Bidus, M. A., Zahn, C., et al. (2007). Overexpression of folate binding protein and mesothelin are associated with uterine serous carcinoma. Gynecologic Oncology, 105(3), 563–570. doi:10.1016/j.ygyno.2006.doi:10.063.
Elnakat, H., & Ratnam, M. (2006). Role of folate receptor genes in reproduction and related cancers. Frontiers in Bioscience, 11, 506–519.
Parker, N., Turk, M. J., Westrick, E., Lewis, J. D., Low, P. S., & Leamon, C. P. (2005). Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Analytical Biochemistry, 338(2), 284–293. doi:10.1016/j.ab.2004.12.026.
Kelemen, L. E. (2006). The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? International Journal of Cancer, 119(2), 243–250. doi:10.1002/ijc.21712.
Peoples, G. E., Anderson, B. W., Fisk, B., Kudelka, A. P., Wharton, J. T., & Ioannides, C. G. (1998). Ovarian cancer-associated lymphocyte recognition of folate binding protein peptides. Annals of Surgical Oncology, 5(8), 743–750.
Peoples, G. E., Anderson, B. W., Lee, T. V., Murray, J. L., Kudelka, A. P., Wharton, J. T., et al. (1999). Vaccine implications of folate binding protein, a novel cytotoxic T lymphocyte-recognized antigen system in epithelial cancers. Clinical Cancer Research, 5(12), 4214–4223.
Tassi, R. A., Calza, S., Ravaggi, A., Bignotti, E., Odicino, F. E., Tognon, G., et al. (2009). Mammaglobin B is an independent prognostic marker in epithelial ovarian cancer and its expression is associated with reduced risk of disease recurrence. BMC Cancer, 9, 253. doi:10.1186/1471-2407-9-253.
Tassi, R. A., Bignotti, E., Falchetti, M., Calza, S., Ravaggi, A., Rossi, E., et al. (2008). Mammaglobin B expression in human endometrial cancer. International Journal of Gynecological Cancer, 18(5), 1090–1096. doi:10.1111/j.1525-1438.2007.01137.x.
Tassi, R. A., Bignotti, E., Rossi, E., Falchetti, M., Donzelli, C., Calza, S., et al. (2007). Overexpression of mammaglobin B in epithelial ovarian carcinomas. Gynecologic Oncology, 105(3), 578–585. doi:10.1016/j.ygyno.2007.01.043.
Bellone, S., Tassi, R., Betti, M., English, D., Cocco, E., Gasparrini, S., et al. (2013). Mammaglobin B (SCGB2A1) is a novel tumour antigen highly differentially expressed in all major histological types of ovarian cancer: Implications for ovarian cancer immunotherapy. British Journal of Cancer, 109(2), 462–471. doi:10.1038/bjc.2013.315.
Pastan, I., & Hassan, R. (2014). Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Research, 74, 2907–2912.
Frierson, H. F., Jr., Moskaluk, C. A., Powell, S. M., Zhang, H., Cerilli, L. A., Stoler, M. H., et al. (2003). Large-scale molecular and tissue microarray analysis of mesothelin expression in common human carcinomas. Human Pathology, 34(6), 605–609.
Ho, M., Hassan, R., Zhang, J., Wang, Q. C., Onda, M., Bera, T., et al. (2005). Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clinical Cancer Research, 11(10), 3814–3820. doi:10.1158/1078-0432.CCR-04-2304.
Cheng, W. F., Huang, C. Y., Chang, M. C., Hu, Y. H., Chiang, Y. C., Chen, Y. L., et al. (2009). High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma. British Journal of Cancer, 100(7), 1144–1153. doi:10.1038/sj.bjc.6604964.
Kalli, K. R. (2007). MORAb-003, a fully humanized monoclonal antibody against the folate receptor alpha, for the potential treatment of epithelial ovarian cancer. Current Opinion in Investigational Drugs, 8(12), 1067–1073.
Ebel, W., Routhier, E. L., Foley, B., Jacob, S., McDonough, J. M., Patel, R. K., et al. (2007). Preclinical evaluation of MORAb-003, a humanized monoclonal antibody antagonizing folate receptor-alpha. Cancer Immunity, 7, 6.
Smith-Jones, P. M., Pandit-Taskar, N., Cao, W., O’Donoghue, J., Philips, M. D., Carrasquillo, J., et al. (2008). Preclinical radioimmunotargeting of folate receptor alpha using the monoclonal antibody conjugate DOTA-MORAb-003. Nuclear Medicine and Biology, 35(3), 343–351. doi:10.1016/j.nucmedbio.2007.12.008.
Armstrong, D. K., Coleman, R., White, A. J., Bicher, A., Gibbon, D. G., Old, L. J., et al. (2009). Efficacy and safety of farletuzumab, a humanized monoclonal antibody to folate receptor alpha, in platinum-sensitive relapsed ovarian cancer subjects: Preliminary data from a phase-2 study. European Journal of Cancer, 7(2 Suppl), 450.
White, A.J., Coleman, R., Armstrong, D.K., Glenn D., Bicher, A., Richards, D.A., et al. (2010). Efficacy and safety of farletuzumab, a humanized monoclonal antibody to folate receptor alpha, in platinum-sensitive relapsed ovarian cancer subjects: Final data from a multicenter phase II study. Journal of Clinical Oncology, 28(15_Suppl).
Spannuth, W. A., Sood, A. K., & Coleman, R. L. (2010). Farletuzumab in epithelial ovarian carcinoma. Expert Opinion on Biological Therapy, 10(3), 431–437. doi:10.1517/14712591003592069.
Hudis, C. A. (2007). Trastuzumab—Mechanism of action and use in clinical practice. The New England Journal of Medicine, 357(1), 39–51. doi:10.1056/NEJMra043186.
Ray-Coquard, I., Guastalla, J. P., Allouache, D., Combe, M., Weber, B., Cretin, J., et al. (2008). HER2 overexpression/amplification and Trastuzumab treatment in advanced ovarian cancer: A GINECO phase II study. Clinical Ovarian Cancer, 1(1), 54–59.
McAlpine, J. N., Wiegand, K. C., Vang, R., Ronnett, B. M., Adamiak, A., Kobel, M., et al. (2009). HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer, 9, 433. doi:10.1186/1471-2407-9-433.
Gordon, M. S., Matei, D., Aghajanian, C., Matulonis, U. A., Brewer, M., Fleming, G. F., et al. (2006). Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: Potential predictive relationship with tumor HER2 activation status. Journal of Clinical Oncology, 24(26), 4324–4332. doi:10.1200/JCO.2005.05.4221.
Takai, N., Jain, A., Kawamata, N., Popoviciu, L. M., Said, J. W., Whittaker, S., et al. (2005). 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer, 104(12), 2701–2708. doi:10.1002/cncr.21533.
Makhija, S., Amler, L. C., Glenn, D., Ueland, F. R., Gold, M. A., Dizon, D. S., et al. (2010). Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. Journal of Clinical Oncology, 28(7), 1215–1223. doi:10.1200/JCO.2009.22.3354.
Kaye, S. B., Poole, C. J., Danska-Bidzinska, A., Gianni, L., Del Conte, G., Gorbunova, V., et al. (2013). A randomized phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. Annals of Oncology, 24(1), 145–152. doi:10.1093/annonc/mds282.
Langdon, S. P., Faratian, D., Nagumo, Y., Mullen, P., & Harrison, D. J. (2010). Pertuzumab for the treatment of ovarian cancer. Expert Opinion on Biological Therapy, 10(7), 1113–1120. doi:10.1517/14712598.20doi:10.487062.
Grisham, R. N., Berek, J., Pfisterer, J., & Sabbatini, P. (2011). Abagovomab: An anti-idiotypic CA-125 targeted immunotherapeutic agent for ovarian cancer. Immunotherapy, 3(2), 153–162. doi:10.2217/imt.doi:10.100.
Wagner, U., Kohler, S., Reinartz S., Giffels P., Huober J., Renke K., et al. (2001). Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti-idiotype antibody ACA125: Immune responses and survival in palliative treatment. See The biology behind: K. A. Foon and M. Bhattacharya-Chatterjee, Are solid tumor anti-idiotype vaccines ready for prime time? Clin. Cancer Res., 7:1112–1115, 2001. Clinical Cancer Research 7(5), 1154–1162.
Sabbatini, P., Harter, P., Scambia, G., Sehouli, J., Meier, W., Wimberger, P., et al. (2013). Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: A phase III trial of the AGO OVAR, COGI, GINECO, and GEICO–the MIMOSA study. Journal of Clinical Oncology, 31(12), 1554–1561. doi:10.1200/JCO.2012.46.4057.
Berek, J., Taylor, P., McGuire, W., Smith, L. M., Schultes, B., & Nicodemus, C. F. (2009). Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer. Journal of Clinical Oncology, 27(3), 418–425. doi:10.1200/JCO.2008.17.8400.
Ehlen, T. G., Hoskins, P. J., Miller, D., Whiteside, T. L., Nicodemus, C. F., Schultes, B. C., et al. (2005). A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer. International Journal of Gynecological Cancer, 15(6), 1023–1034. doi:10.1111/j.1525-1438.2005.00483.x.
Berek, J. S., Taylor, P. T., Gordon, A., Cunningham, M. J., Finkler, N., Orr, J., Jr., et al. (2004). Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. Journal of Clinical Oncology, 22(17), 3507–3516. doi:10.1200/JCO.2004.09.016.
Ruf, P., Gires, O., Jager, M., Fellinger, K., Atz, J., & Lindhofer, H. (2007). Characterisation of the new EpCAM-specific antibody HO-3: Implications for trifunctional antibody immunotherapy of cancer. British Journal of Cancer, 97(3), 315–321. doi:10.1038/sj.bjc.6603881.
Burges, A., Wimberger, P., Kumper, C., Gorbounova, V., Sommer, H., Schmalfeldt, B., et al. (2007). Effective relief of malignant ascites in patients with advanced ovarian cancer by a trifunctional anti-EpCAM x anti-CD3 antibody: A phase I/II study. Clinical Cancer Research, 13(13), 3899–3905. doi:10.1158/1078-0432.CCR-06-2769.
Heiss, M. M., Murawa, P., Koralewski, P., Kutarska, E., Kolesnik, O. O., Ivanchenko, V. V., et al. (2010). The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. International Journal of Cancer, 127(9), 2209–2221. doi:10.1002/ijc.25423.
Baumann, K., Pfisterer, J., Wimberger, P., Burchardi, N., Kurzeder, C., du Bois, A., et al. (2011). Intraperitoneal treatment with the trifunctional bispecific antibody catumaxomab in patients with platinum-resistant epithelial ovarian cancer: A phase IIa study of the AGO study group. Gynecologic Oncology, 123(1), 27–32. doi:10.1016/j.ygyno.2011.06.004.
Rossi, J. F., Negrier, S., James, N. D., Kocak, I., Hawkins, R., Davis, H., et al. (2010). A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. British Journal of Cancer, 103(8), 1154–1162. doi:10.1038/sj.bjc.6605872.
Karkera, J., Steiner, H., Li, W., Skradski, V., Moser, P. L., Riethdorf, S., et al. (2011). The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. The Prostate, 71(13), 1455–1465. doi:10.1002/pros.21362.
Fizazi, K., De Bono, J. S., Flechon, A., Heidenreich, A., Voog, E., Davis, N. B., et al. (2012). Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. European Journal of Cancer, 48(1), 85–93. doi:10.1016/j.ejca.2011.doi:10.014.
Dorff, T. B., Goldman, B., Pinski, J. K., Mack, P. C., Lara, P. N., Jr., Van Veldhuizen, P. J., Jr., et al. (2010). Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clinical Cancer Research, 16(11), 3028–3034. doi:10.1158/1078-0432.CCR-09-3122.
Angevin, E., Tabernero, J., Elez, E., Cohen, S. J., Bahleda, R., van Laethem, J. L., et al. (2014). A phase I/II, multiple-dose, dose-escalation study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with advanced solid tumors. Clinical Cancer Research, 20(8), 2192–2204. doi:10.1158/1078-0432.CCR-13-2200.
Hodi, F. S., Mihm, M. C., Soiffer, R. J., Haluska, F. G., Butler, M., Seiden, M. V., et al. (2003). Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proceedings of the National Academy of Sciences of the United States of America, 100(8), 4712–4717. doi:10.1073/pnas.0830997100.
Brahmer, J. R., Tykodi, S. S., Chow, L. Q., Hwu, W. J., Topalian, S. L., Hwu, P., et al. (2012). Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. The New England Journal of Medicine, 366(26), 2455–2465. doi:10.1056/NEJMoa1200694.
Knutson, K. L., Wagner, W., & Disis, M. L. (2006). Adoptive T cell therapy of solid cancers. Cancer Immunology, Immunotherapy, 55(1), 96–103. doi:10.1007/s00262-005-0706-1.
Kalos, M., & June, C. H. (2013). Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology. Immunity, 39(1), 49–60. doi:10.1016/j.immuni.2013.07.002.
June, C. H. (2007). Adoptive T cell therapy for cancer in the clinic. The Journal of Clinical Investigation, 117(6), 1466–1476. doi:10.1172/JCI32446.
June, C. H. (2007). Principles of adoptive T cell cancer therapy. The Journal of Clinical Investigation, 117(5), 1204–1212. doi:10.1172/JCI31446.
Rosenberg, S. A., & Dudley, M. E. (2009). Adoptive cell therapy for the treatment of patients with metastatic melanoma. Current Opinion in Immunology, 21(2), 233–240. doi:10.1016/j.coi.2009.03.002.
Kershaw, M. H., Westwood, J. A., Parker, L. L., Wang, G., Eshhar, Z., Mavroukakis, S. A., et al. (2006). A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical Cancer Research, 12(20 Pt 1), 6106–6115. doi:10.1158/1078-0432.CCR-06-1183.
Dobrzanski, M. J., Rewers-Felkins, K. A., Quinlin, I. S., Samad, K. A., Phillips, C. A., Robinson, W., et al. (2009). Autologous MUC1-specific Th1 effector cell immunotherapy induces differential levels of systemic TReg cell subpopulations that result in increased ovarian cancer patient survival. Clinical Immunology, 133(3), 333–352. doi:10.1016/j.clim.2009.08.007.
Dobrzanski, M. J., Rewers-Felkins, K. A., Samad, K. A., Quinlin, I. S., Phillips, C. A., Robinson, W., et al. (2012). Immunotherapy with IL-10- and IFN-gamma-producing CD4 effector cells modulate “natural” and “inducible” CD4 TReg cell subpopulation levels: Observations in four cases of patients with ovarian cancer. Cancer Immunology, Immunotherapy, 61(6), 839–854. doi:10.1007/s00262-011-1128-x.
Wright, S. E., Rewers-Felkins, K. A., Quinlin, I. S., Phillips, C. A., Townsend, M., Philip, R., et al. (2012). Cytotoxic T-lymphocyte immunotherapy for ovarian cancer: A pilot study. Journal of Immunotherapy, 35(2), 196–204. doi:10.1097/CJI.0b013e318243f213.
Le, D. T., Brockstedt, D. G., Nir-Paz, R., Hampl, J., Mathur, S., Nemunaitis, J., et al. (2012). A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: Phase I studies of safety and immune induction. Clinical Cancer Research, 18(3), 858–868. doi:10.1158/1078-0432.CCR-11-2121.
Melief, C. J., & van der Burg, S. H. (2008). Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nature Reviews Cancer, 8(5), 351–360. doi:10.1038/nrc2373.
Sabbatini, P., Tsuji, T., Ferran, L., Ritter, E., Sedrak, C., Tuballes, K., et al. (2012). Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clinical Cancer Research, 18(23), 6497–6508. doi:10.1158/1078-0432.CCR-12-2189.
Kaumaya, P. T., Foy, K. C., Garrett, J., Rawale, S. V., Vicari, D., Thurmond, J. M., et al. (2009). Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. Journal of Clinical Oncology, 27(31), 5270–5277. doi:10.1200/JCO.2009.22.3883.
Hernando, J. J., Park, T. W., Fischer, H. P., Zivanovic, O., Braun, M., Polcher, M., et al. (2007). Vaccination with dendritic cells transfected with mRNA-encoded folate-receptor-alpha for relapsed metastatic ovarian cancer. The Lancet Oncology, 8(5), 451–454. doi:10.1016/S1470-2045(07)70142-0.
Zum Buschenfelde, C. M., Hermann, C., Schmidt, B., Peschel, C., & Bernhard, H. (2002). Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Research, 62(8), 2244–2247.
Disis, M. L., Wallace, D. R., Gooley, T. A., Dang, Y., Slota, M., Lu, H., et al. (2009). Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. Journal of Clinical Oncology, 27(28), 4685–4692. doi:10.1200/JCO.2008.20.6789.
Muraoka-Cook, R. S., Dumont, N., & Arteaga, C. L. (2005). Dual role of transforming growth factor beta in mammary tumorigenesis and metastatic progression. Clinical Cancer Research, 11(2 Pt 2), 937s–943s.
Halder, S. K., Beauchamp, R. D., & Datta, P. K. (2005). A specific inhibitor of TGF-beta receptor kinase, SB-431542, as a potent antitumor agent for human cancers. Neoplasia, 7(5), 509–521.
von Boehmer, H. (2005). Mechanisms of suppression by suppressor T cells. Nature Immunology, 6(4), 338–344. doi:10.1038/ni1180.
Acknowledgments
This work is supported by P50-CA136393 (KLK), a grant from the Marsha Rivkin Center for Ovarian Cancer Research and support from the Mayo Graduate School (PL). The authors gratefully acknowledge the expert editorial assistance of Shaundia White of the Vaccine and Gene Therapy Institute of Florida.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article has been retracted at the request of the authors and in agreement with the Editors in Chief. The article contains large portions of text that have been duplicated from the articles:
1. Immunity and immune suppression in human ovarian cancer Claudia C Preston, Ellen L Goode, Lynn C Hartmann, Kimberly R Kalli, and Keith L Knutson Immunotherapy. 2011 Apr. 3(4): 539–556, DOI 10.2217/imt.11.20
2. The Immune System in the Pathogenesis of Ovarian Cancer Bridget Charbonneau, Ellen L. Goode, Kimberly R. Kalli, Keith L. Knutson, and Melissa S. DeRycke Crit Rev Immunol. 2013; 33(2): 137–164, DOI: 10.1615/CritRevImmunol.2013006813
An erratum to this article can be found online at http://dx.doi.org/10.1007/s10555-016-9627-z.
About this article
Cite this article
Knutson, K.L., Karyampudi, L., Lamichhane, P. et al. RETRACTED ARTICLE: Targeted immune therapy of ovarian cancer. Cancer Metastasis Rev 34, 53–74 (2015). https://doi.org/10.1007/s10555-014-9540-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9540-2