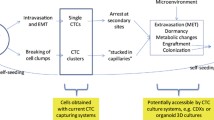
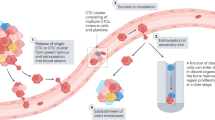
Abstract
Circulating tumour cells (CTCs) are emerging as important prognostic markers and have potential clinical utility as tumour biomarkers for targeted cancer therapy. Although CTCs were proposed more than 100 years ago as potential precursors that may form metastatic lesions, formal evidence that CTCs are indeed capable of initiating metastases is limited. Moreover, the process of CTCs shedding into the circulation, relocating to distant organ sites and initiating metastatic foci is complex and intrinsically inefficient. To partially explain the metastatic process, the concepts of CTCs as metastatic precursors or pre-metastatic conditioners have been proposed; however, it is questionable as to whether these are both variable pathways to metastasis or just markers of metastatic burden. This review explores the evidence for CTCs in the initiation and progression of metastatic cancer and the data supporting these different concepts in an attempt to better understand the role of CTCs in metastasis. A greater understanding of the metastatic potential of CTCs will open new avenues for therapeutic interventions in the future.

Similar content being viewed by others
References
Cohen, S. J., Punt, C. J., Iannotti, N., Saidman, B. H., Sabbath, K. D., Gabrail, N. Y., et al. (2009). Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Annals of Oncology, 20(7), 1223–1229. doi:10.1093/annonc/mdn786.
Cristofanilli, M., Budd, G. T., Ellis, M. J., Stopeck, A., Matera, J., Miller, M. C., et al. (2004). Circulating tumor cells, disease progression, and survival in metastatic breast cancer. New England Journal of Medicine, 351(8), 781–791. doi:10.1056/NEJMoa040766.
Pierga, J. Y., Hajage, D., Bachelot, T., Delaloge, S., Brain, E., Campone, M., et al. (2012). High independent prognostic and predictive value of circulating tumor cells compared with serum tumor markers in a large prospective trial in first-line chemotherapy for metastatic breast cancer patients. Annals of Oncology, 23(3), 618–624. doi:10.1093/annonc/mdr263.
Thalgott, M., Rack, B., Maurer, T., Souvatzoglou, M., Eiber, M., Kress, V., et al. (2013). Detection of circulating tumor cells in different stages of prostate cancer. Journal of Cancer Research and Clinical Oncology, 139(5), 755–763. doi:10.1007/s00432-013-1377-5.
Hayes, D. F., Cristofanilli, M., Budd, G. T., Ellis, M. J., Stopeck, A., Miller, M. C., et al. (2006). Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clinical Cancer Research, 12(14 Pt 1), 4218–4224. doi:10.1158/1078-0432.CCR-05-2821.
Scher, H. I., Jia, X., de Bono, J. S., Fleisher, M., Pienta, K. J., Raghavan, D., et al. (2009). Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncology, 10(3), 233–239. doi:10.1016/S1470-2045(08)70340-1.
Dong, X., Alpaugh, K. R., & Cristofanilli, M. (2012). Circulating tumor cells (CTCs) in breast cancer: a diagnostic tool for prognosis and molecular analysis. Chinese Journal of Cancer Research, 24(4), 388–398. doi:10.3978/j.issn.1000-9604.2012.11.03.
Khan, M. S., Kirkwood, A., Tsigani, T., Garcia-Hernandez, J., Hartley, J. A., Caplin, M. E., et al. (2013). Circulating tumor cells as prognostic markers in neuroendocrine tumors. Journal of Clinical Oncology, 31(3), 365–372. doi:10.1200/JCO.2012.44.2905.
Giuliano, M., Giordano, A., Jackson, S., Hess, K. R., De Giorgi, U., Mego, M., et al. (2011). Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Research, 13(3), R67. doi:10.1186/bcr2907.
Lucci, A., Hall, C. S., Lodhi, A. K., Bhattacharyya, A., Anderson, A. E., Xiao, L., et al. (2012). Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncology, 13(7), 688–695. doi:10.1016/S1470-2045(12)70209-7.
Aggarwal, C., Meropol, N. J., Punt, C. J., Iannotti, N., Saidman, B. H., Sabbath, K. D., et al. (2013). Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Annals of Oncology, 24(2), 420–428. doi:10.1093/annonc/mds336.
Miyamoto, D. T., Lee, R. J., Stott, S. L., Ting, D. T., Wittner, B. S., Ulman, M., et al. (2012). Androgen receptor signaling in circulating tumor cells as a marker of hormonally responsive prostate cancer. Cancer Discovery, 2(11), 995–1003. doi:10.1158/2159-8290.CD-12-0222.
Ashworth, T. R. (1869). A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Australian Medical Journal, 14, 146–147.
Valastyan, S., & Weinberg, R. A. (2011). Tumor metastasis: molecular insights and evolving paradigms. Cell, 147(2), 275–292. doi:10.1016/j.cell.2011.09.024.
Fidler, I. J. (2002). The organ microenvironment and cancer metastasis. Differentiation, 70(9–10), 498–505. doi:10.1046/j.1432-0436.2002.700904.x.
Paget, S. (1889). The distribution of secondary growths in cancer of the breast. Lancet, 1, 571–573.
Baccelli, I., Schneeweiss, A., Riethdorf, S., Stenzinger, A., Schillert, A., Vogel, V., et al. (2013). Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nature Biotechnology, 31(6), 539–544. doi:10.1038/nbt.2576.
Meng, S., Tripathy, D., Frenkel, E. P., Shete, S., Naftalis, E. Z., Huth, J. F., et al. (2004). Circulating tumor cells in patients with breast cancer dormancy. Clinical Cancer Research, 10(24), 8152–8162. doi:10.1158/1078-0432.CCR-04-1110.
Tiwari, N., Gheldof, A., Tatari, M., & Christofori, G. (2012). EMT as the ultimate survival mechanism of cancer cells. Seminars in Cancer Biology, 22(3), 194–207. doi:10.1016/j.semcancer.2012.02.013.
Nieto, M. A., & Cano, A. (2012). The epithelial-mesenchymal transition under control: global programs to regulate epithelial plasticity. Seminars in Cancer Biology, 22(5–6), 361–368. doi:10.1016/j.semcancer.2012.05.003.
Lindemann, F., Schlimok, G., Dirschedl, P., Witte, J., & Riethmuller, G. (1992). Prognostic significance of micrometastatic tumour cells in bone marrow of colorectal cancer patients. Lancet, 340(8821), 685–689.
Vogel, P., Ruschoff, J., Kummel, S., Zirngibl, H., Hofstadter, F., Hohenberger, W., et al. (2000). Prognostic value of microscopic peritoneal dissemination: comparison between colon and gastric cancer. Diseases of the Colon and Rectum, 43(1), 92–100.
de Bono, J. S., Scher, H. I., Montgomery, R. B., Parker, C., Miller, M. C., Tissing, H., et al. (2008). Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical Cancer Research, 14(19), 6302–6309. doi:10.1158/1078-0432.CCR-08-0872.
De Giorgi, U., Mego, M., Scarpi, E., Giuliano, M., Giordano, A., Reuben, J. M., et al. (2012). Relationship between lymphocytopenia and circulating tumor cells as prognostic factors for overall survival in metastatic breast cancer. Clinical Breast Cancer, 12(4), 264–269. doi:10.1016/j.clbc.2012.04.004.
Hiltermann, T. J., Pore, M. M., van den Berg, A., Timens, W., Boezen, H. M., Liesker, J. J., et al. (2012). Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Annals of Oncology, 23(11), 2937–2942. doi:10.1093/annonc/mds138.
Hou, J. M., Krebs, M. G., Lancashire, L., Sloane, R., Backen, A., Swain, R. K., et al. (2012). Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. Journal of Clinical Oncology, 30(5), 525–532. doi:10.1200/JCO.2010.33.3716.
Nieva, J., Wendel, M., Luttgen, M. S., Marrinucci, D., Bazhenova, L., Kolatkar, A., et al. (2012). High-definition imaging of circulating tumor cells and associated cellular events in non-small cell lung cancer patients: a longitudinal analysis. Physical Biology, 9(1), 016004. doi:10.1088/1478-3975/9/1/016004.
Luzzi, K. J., MacDonald, I. C., Schmidt, E. E., Kerkvliet, N., Morris, V. L., Chambers, A. F., et al. (1998). Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. American Journal of Pathology, 153(3), 865–873. doi:10.1016/S0002-9440(10)65628-3.
Mervic, L. (2012). Time course and pattern of metastasis of cutaneous melanoma differ between men and women. PLoS ONE, 7(3), e32955. doi:10.1371/journal.pone.0032955.
Tas, F. (2012). Factors influencing the hormone receptor and HER2 levels in breast cancer: a population-based analysis. Onkologie, 35(3), 95–98. doi:10.1159/000336812.
Coumans, F. A., Siesling, S., & Terstappen, L. W. (2013). Detection of cancer before distant metastasis. BMC Cancer, 13(1), 283. doi:10.1186/1471-2407-13-283.
Ito, S., Nakanishi, H., Ikehara, Y., Kato, T., Kasai, Y., Ito, K., et al. (2001). Real-time observation of micrometastasis formation in the living mouse liver using a green fluorescent protein gene-tagged rat tongue carcinoma cell line. International Journal of Cancer, 93(2), 212–217. doi:10.1002/ijc.1318.
Weiss, L. (2000). Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer and Metastasis Reviews, 19(3-4), I–XI. 193-383.
Becker, T. M., Caixeiro, N. J., Lim, S. H., Tognela, A., Kienzle, N., Scott, K. F., et al. (2014). New frontiers in circulating tumor cell analysis: a reference guide for biomolecular profiling toward translational clinical use. International Journal of Cancer, 134(11), 2523–2533. doi:10.1002/ijc.28516.
Rossi, E., Basso, U., Celadin, R., Zilio, F., Pucciarelli, S., Aieta, M., et al. (2010). M30 neoepitope expression in epithelial cancer: quantification of apoptosis in circulating tumor cells by cell search analysis. Clinical Cancer Research, 16(21), 5233–5243. doi:10.1158/1078-0432.CCR-10-1449.
Larson, C. J., Moreno, J. G., Pienta, K. J., Gross, S., Repollet, M., & O’Hara, S. M. (2004). Apoptosis of circulating tumor cells in prostate cancer patients. Cytometry Part A, 62(1), 46–53. doi:10.1002/cyto.a.20073.
Powell, A. A., Talasaz, A. H., Zhang, H., Coram, M. A., Reddy, A., Deng, G., et al. (2012). Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE, 7(5), e33788. doi:10.1371/journal.pone.0033788.
Duda, D. G., Duyverman, A. M., Kohno, M., Snuderl, M., Steller, E. J., Fukumura, D., et al. (2010). Malignant cells facilitate lung metastasis by bringing their own soil. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21677–21682. doi:10.1073/pnas.1016234107.
Joyce, J. A., & Pollard, J. W. (2009). Microenvironmental regulation of metastasis. Nature Reviews Cancer, 9(4), 239–252. doi:10.1038/nrc2618.
Honn, K. V., Tang, D. G., & Crissman, J. D. (1992). Platelets and cancer metastasis: a causal relationship? Cancer and Metastasis Reviews, 11(3–4), 325–351.
Palumbo, J. S., Talmage, K. E., Massari, J. V., La Jeunesse, C. M., Flick, M. J., Kombrinck, K. W., et al. (2005). Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood, 105(1), 178–185. doi:10.1182/blood-2004-06-2272.
Erpenbeck, L., Nieswandt, B., Schon, M., Pozgajova, M., & Schon, M. P. (2010). Inhibition of platelet GPIb alpha and promotion of melanoma metastasis. Journal of Investigative Dermatology, 130(2), 576–586. doi:10.1038/jid.2009.278.
Hafner, M., Orosz, P., Kruger, A., & Mannel, D. N. (1996). TNF promotes metastasis by impairing natural killer cell activity. International Journal of Cancer, 66(3), 388–392. doi:10.1002/(SICI)1097-0215(19960503)66:3<388::AID-IJC20>3.0.CO;2-6.
Steinert, G., Scholch, S., Niemietz, T., Iwata, N., Garcia, S. A., Behrens, B., et al. (2014). Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Research, 74(6), 1694–1704. doi:10.1158/0008-5472.CAN-13-1885.
Shao, B., Wahrenbrock, M. G., Yao, L., David, T., Coughlin, S. R., Xia, L., et al. (2011). Carcinoma mucins trigger reciprocal activation of platelets and neutrophils in a murine model of Trousseau syndrome. Blood, 118(15), 4015–4023. doi:10.1182/blood-2011-07-368514.
Letai, A., & Kuter, D. J. (1999). Cancer, coagulation, and anticoagulation. The Oncologist, 4(6), 443–449.
Micalizzi, D. S., Farabaugh, S. M., & Ford, H. L. (2010). Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. Journal of Mammary Gland Biology and Neoplasia, 15(2), 117–134. doi:10.1007/s10911-010-9178-9.
Cano, A., Perez-Moreno, M. A., Rodrigo, I., Locascio, A., Blanco, M. J., del Barrio, M. G., et al. (2000). The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biology, 2(2), 76–83. doi:10.1038/35000025.
Talbot, L. J., Bhattacharya, S. D., & Kuo, P. C. (2012). Epithelial-mesenchymal transition, the tumor microenvironment, and metastatic behavior of epithelial malignancies. International Journal of Biochemistry and Molecular Biology, 3(2), 117–136.
Sieuwerts, A. M., Mostert, B., Bolt-de Vries, J., Peeters, D., de Jongh, F. E., Stouthard, J. M., et al. (2011). mRNA and microRNA expression profiles in circulating tumor cells and primary tumors of metastatic breast cancer patients. Clinical Cancer Research, 17(11), 3600–3618. doi:10.1158/1078-0432.CCR-11-0255.
Yokobori, T., Iinuma, H., Shimamura, T., Imoto, S., Sugimachi, K., Ishii, H., et al. (2013). Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Research, 73(7), 2059–2069. doi:10.1158/0008-5472.CAN-12-0326.
Kallergi, G., Papadaki, M. A., Politaki, E., Mavroudis, D., Georgoulias, V., & Agelaki, S. (2011). Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Research, 13(3), R59. doi:10.1186/bcr2896.
Balasubramanian, P., Lang, J. C., Jatana, K. R., Miller, B., Ozer, E., Old, M., et al. (2012). Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS ONE, 7(7), e42048. doi:10.1371/journal.pone.0042048.
Becker, T. M., Caixeiro, N. J., Lim, S. H., Tognela, A., Kienzle, N., Scott, K. F., et al. (2013). New frontiers in circulating tumor cell analysis—a reference guide for biomolecular profiling towards translational clinical use. International Journal of Cancer. doi:10.1002/ijc.28516.
Giordano, A., Gao, H., Anfossi, S., Cohen, E., Mego, M., Lee, B. N., et al. (2012). Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Molecular Cancer Therapeutics, 11(11), 2526–2534. doi:10.1158/1535-7163.MCT-12-0460.
Mego, M., Mani, S. A., Lee, B. N., Li, C., Evans, K. W., Cohen, E. N., et al. (2012). Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: the effect of neoadjuvant therapy. International Journal of Cancer, 130(4), 808–816. doi:10.1002/ijc.26037.
Satelli, A., Mitra, A., Cutrera, J. J., Devarie, M., Xia, X., Ingram, D. R., et al. (2014). Universal marker and detection tool for human sarcoma circulating tumor cells. Cancer Research, 74(6), 1645–1650. doi:10.1158/0008-5472.CAN-13-1739.
Muller, A., Homey, B., Soto, H., Ge, N., Catron, D., Buchanan, M. E., et al. (2001). Involvement of chemokine receptors in breast cancer metastasis. Nature, 410(6824), 50–56. doi:10.1038/35065016.
Craig, M. J., & Loberg, R. D. (2006). CCL2 (monocyte chemoattractant protein-1) in cancer bone metastases. Cancer and Metastasis Reviews, 25(4), 611–619. doi:10.1007/s10555-006-9027-x.
Thiery, J. P. (2003). Epithelial-mesenchymal transitions in development and pathologies. Current Opinion in Cell Biology, 15(6), 740–746.
Chambers, A. F., Groom, A. C., & MacDonald, I. C. (2002). Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer, 2(8), 563–572. doi:10.1038/nrc865.
Pantel, K., & Brakenhoff, R. H. (2004). Dissecting the metastatic cascade. Nature Reviews Cancer, 4(6), 448–456. doi:10.1038/nrc1370.
Chiang, A. C., & Massague, J. (2008). Molecular basis of metastasis. New England Journal of Medicine, 359(26), 2814–2823. doi:10.1056/NEJMra0805239.
Nierodzik, M. L., & Karpatkin, S. (2006). Thrombin induces tumor growth, metastasis, and angiogenesis: evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell, 10(5), 355–362. doi:10.1016/j.ccr.2006.10.002.
Gantus, M. A., Alves, L. M., Stipursky, J., Souza, E. C., Teodoro, A. J., Alves, T. R., et al. (2011). Estradiol modulates TGF-beta1 expression and its signaling pathway in thyroid stromal cells. Molecular and Cellular Endocrinology, 337(1–2), 71–79. doi:10.1016/j.mce.2011.02.001.
Heitzer, E., Auer, M., Gasch, C., Pichler, M., Ulz, P., Hoffmann, E. M., et al. (2013). Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Research, 73(10), 2965–2975. doi:10.1158/0008-5472.CAN-12-4140.
Smirnov, D. A., Zweitzig, D. R., Foulk, B. W., Miller, M. C., Doyle, G. V., Pienta, K. J., et al. (2005). Global gene expression profiling of circulating tumor cells. Cancer Research, 65(12), 4993–4997. doi:10.1158/0008-5472.CAN-04-4330.
Shaikhibrahim, Z., Lindstrot, A., Ellinger, J., Rogenhofer, S., Buettner, R., & Wernert, N. (2012). Genes differentially expressed in the peripheral zone compared to the transitional zone of the normal human prostate and their potential regulation by ETS factors. Molecular Medicine Reports, 5(1), 32–36. doi:10.3892/mmr.2011.628.
Raja, S. B., Murali, M. R., Devaraj, H., & Devaraj, S. N. (2012). Differential expression of gastric MUC5AC in colonic epithelial cells: TFF3-wired IL1 beta/Akt crosstalk-induced mucosal immune response against Shigella dysenteriae infection. Journal of Cell Science, 125(Pt 3), 703–713. doi:10.1242/jcs.092148.
Bidard, F. C., Pierga, J. Y., Vincent-Salomon, A., & Poupon, M. F. (2008). A “class action” against the microenvironment: do cancer cells cooperate in metastasis? Cancer and Metastasis Reviews, 27(1), 5–10. doi:10.1007/s10555-007-9103-x.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144(5), 646–674. doi:10.1016/j.cell.2011.02.013.
Vanderlaag, K. E., Hudak, S., Bald, L., Fayadat-Dilman, L., Sathe, M., Grein, J., et al. (2010). Anterior gradient-2 plays a critical role in breast cancer cell growth and survival by modulating cyclin D1, estrogen receptor-alpha and survivin. Breast Cancer Research, 12(3), R32. doi:10.1186/bcr2586.
Khatib, A. M., Auguste, P., Fallavollita, L., Wang, N., Samani, A., Kontogiannea, M., et al. (2005). Characterization of the host proinflammatory response to tumor cells during the initial stages of liver metastasis. American Journal of Pathology, 167(3), 749–759. doi:10.1016/S0002-9440(10)62048-2.
Auguste, P., Fallavollita, L., Wang, N., Burnier, J., Bikfalvi, A., & Brodt, P. (2007). The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. American Journal of Pathology, 170(5), 1781–1792. doi:10.2353/ajpath.2007.060886.
Liotta, L. A., & Kohn, E. C. (2001). The microenvironment of the tumour-host interface. Nature, 411(6835), 375–379. doi:10.1038/35077241.
Obermayr, E., Castillo-Tong, D. C., Pils, D., Speiser, P., Braicu, I., Van Gorp, T., et al. (2013). Molecular characterization of circulating tumor cells in patients with ovarian cancer improves their prognostic significance—a study of the OVCAD consortium. Gynecologic Oncology, 128(1), 15–21. doi:10.1016/j.ygyno.2012.09.021.
Bidard, F. C., Pierga, J. Y., Soria, J. C., & Thiery, J. P. (2013). Translating metastasis-related biomarkers to the clinic–progress and pitfalls. Nature Reviews Clinical Oncology, 10(3), 169–179. doi:10.1038/nrclinonc.2013.4.
Zhe, X., Cher, M. L., & Bonfil, R. D. (2011). Circulating tumor cells: finding the needle in the haystack. American Journal of Cancer Research, 1(6), 740–751.
Braun, S., Vogl, F. D., Naume, B., Janni, W., Osborne, M. P., Coombes, R. C., et al. (2005). A pooled analysis of bone marrow micrometastasis in breast cancer. New England Journal of Medicine, 353(8), 793–802. doi:10.1056/NEJMoa050434.
Peinado, H., Aleckovic, M., Lavotshkin, S., Matei, I., Costa-Silva, B., Moreno-Bueno, G., et al. (2012). Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature Medicine, 18(6), 883–891. doi:10.1038/nm.2753.
Pantel, K., Schlimok, G., Kutter, D., Schaller, G., Genz, T., Wiebecke, B., et al. (1991). Frequent down-regulation of major histocompatibility class I antigen expression on individual micrometastatic carcinoma cells. Cancer Research, 51(17), 4712–4715.
Schlimok, G., & Riethmuller, G. (1990). Detection, characterization and tumorigenicity of disseminated tumor cells in human bone marrow. Seminars in Cancer Biology, 1(3), 207–215.
Pantel, K., Schlimok, G., Angstwurm, M., Passlick, B., Izbicki, J. R., Johnson, J. P., et al. (1995). Early metastasis of human solid tumours: expression of cell adhesion molecules. Ciba Foundation Symposium, 189, 157–170. discussion 170-153, 174-156.
Dawson, M. R., Duda, D. G., Fukumura, D., & Jain, R. K. (2009). VEGFR1-activity-independent metastasis formation. Nature, 461(7262), E4. doi:10.1038/nature08254. discussion E5.
Brandt, B., Junker, R., Griwatz, C., Heidl, S., Brinkmann, O., Semjonow, A., et al. (1996). Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Research, 56(20), 4556–4561.
Al-Mehdi, A. B., Tozawa, K., Fisher, A. B., Shientag, L., Lee, A., & Muschel, R. J. (2000). Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nature Medicine, 6(1), 100–102. doi:10.1038/71429.
Gerlinger, M., Rowan, A. J., Horswell, S., Larkin, J., Endesfelder, D., Gronroos, E., et al. (2012). Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. New England Journal of Medicine, 366(10), 883–892. doi:10.1056/NEJMoa1113205.
Gasch, C., Bauernhofer, T., Pichler, M., Langer-Freitag, S., Reeh, M., Seifert, A. M., et al. (2013). Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clinical Chemistry, 59(1), 252–260. doi:10.1373/clinchem.2012.188557.
Niikura, N., Liu, J., Hayashi, N., Mittendorf, E. A., Gong, Y., Palla, S. L., et al. (2012). Loss of human epidermal growth factor receptor 2 (HER2) expression in metastatic sites of HER2-overexpressing primary breast tumors. Journal of Clinical Oncology, 30(6), 593–599. doi:10.1200/JCO.2010.33.8889.
Dupont Jensen, J., Laenkholm, A. V., Knoop, A., Ewertz, M., Bandaru, R., Liu, W., et al. (2011). PIK3CA mutations may be discordant between primary and corresponding metastatic disease in breast cancer. Clinical Cancer Research, 17(4), 667–677. doi:10.1158/1078-0432.CCR-10-1133.
Acknowledgments
This work was supported by the University of Western Sydney and the University of New South Wales. Funding sources include the Cancer Institute New South Wales for the South West Sydney Translational Cancer Research Unit, University of New South Wales Equipment Grant and Prostate Cancer Foundation of Australia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caixeiro, N.J., Kienzle, N., Lim, S.H. et al. Circulating tumour cells—a bona fide cause of metastatic cancer. Cancer Metastasis Rev 33, 747–756 (2014). https://doi.org/10.1007/s10555-014-9502-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9502-8