Abstract
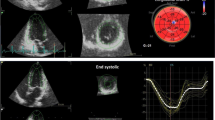
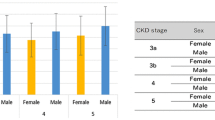
Cardiovascular disease (CVD) is the leading cause of end-stage mortality in chronic kidney disease (CKD) patients. However, CVD and CKD are inextricably linked, as microalbuminuria is an independent risk factor for CVD. Herein, we investigated changes in cardiac function and its risk factors in CKD patients who had different urine albumin-to-creatinine ratios (UACRs) and estimated glomerular filtration rates (eGFRs). We prospectively enrolled 182 CKD patients, classified into three groups based on UACRs and eGFRs. Fifty healthy volunteers were included as controls. Changes in clinical and echocardiographic parameters were assessed in each group, and factors independently associated with strain parameters were further analyzed. Compared with those in the control group, the albuminuria but unimpaired renal function (ALB-CKD G1-2), albuminuria and impaired renal function (ALB-CKD G3), and normoalbuminuric CKD (NACKD) groups had decreased left ventricular (LV), right ventricular (RV), and left atrial (LA) strains, the LA contractile strain being the only statistically comparable parameter. Stepwise multiple linear regression analysis revealed varying factors independently correlating with the LV global longitudinal strain. The LA reservoir and conduit strains independently correlated with LV diastolic function in stage 3 CKD associated with comorbid albuminuria or normoalbuminuria. LV function was a partial determinant of LA and RV function in the ALB-CKD G3 group, whereas ventricular and atrial function were independent of each other in the ALB-CKD G1-2 and NACKD groups. Clinical intervention should focus on specific factors affecting cardiac function in patients to reduce the risk of CVD-related death.






Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author upon reasonable request.
References
Matsushita K, Ballew SH, Wang AY et al (2022) Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol 18:696–707
Patel N, Yaqoob MM, Aksentijevic D (2022) Cardiac metabolic remodelling in chronic kidney disease. Nat Rev Nephrol 18:524–537
Parfrey PS, Foley RN, Harnett JD et al (1996) Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant 11:1277–1285
Zoccali C, Benedetto FA, Mallamaci F et al (2001) Prognostic impact of the indexation of left ventricular mass in patients undergoing dialysis. J Am Soc Nephrol 12:2768–2774
Middleton RJ, Parfrey PS, Foley RN (2001) Left ventricular hypertrophy in the renal patient. J Am Soc Nephrol 12:1079–1084
Zoccali C, Mallamaci F, Tripepi G et al (2002) Norepinephrine and concentric hypertrophy in patients with end-stage renal disease. Hypertension 40:41–46
Zoccali C, Mallamaci F, Maas R et al (2002) Left ventricular hypertrophy, cardiac remodeling and asymmetric dimethylarginine (ADMA) in hemodialysis patients. Kidney Int 62:339–345
Simpson P (1983) Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest 72:732–738
Zoccali C, Benedetto FA, Mallamaci F et al (2004) Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int 65:1492–1498
Cao Y, Sun XY, Zhong M et al (2019) Evaluation of hemodynamics in patients with hypertrophic cardiomyopathy by vector flow mapping: comparison with healthy subjects. Exp Ther Med 17:4379–4388
Wu D, Xuan Y, Ruan Y et al (2016) Prevalence of macro- and microvascular complications in patients with type 2 diabetes and kidney disease with or without albuminuria in a single Chinese diabetes centre. Diab Vasc Dis Res 13:21–30
Penno G, Solini A, Bonora E et al (2011) Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 29:1802–1809
Nohara A (2016) Epicardial adipose tissue as a predictor of plaque vulnerability in patients with mild chronic kidney disease. Circ J 2016;80:64 – 6
Hassan M, Said K, Rizk H et al (2016) Segmental peri-coronary epicardial adipose tissue volume and coronary plaque characteristics. Eur Heart J Cardiovasc Imaging 17:1169–1177
Kadappu KK, Abhayaratna K, Boyd A et al (2016) Independent echocardiographic markers of cardiovascular involvement in chronic kidney disease: the value of left atrial function and volume. J Am Soc Echocardiog 29:359–367
Romejko K, Rymarz A, Szamotulska K et al (2022) Serum osteoprotegerin is an independent marker of left ventricular hypertrophy, systolic and diastolic dysfunction of the left ventricle and the presence of pericardial fluid in chronic kidney disease patients. Nutrients 14:2893
Gan GCH, Kadappu KK, Bhat A et al (2021) Left atrial strain is the best predictor of adverse cardiovascular outcomes in patients with chronic kidney disease. J Am Soc Echocardiog 34:166–175
Levey AS, Eckardt KU, Dorman NM et al (2020) Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 97:1117-29
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–612
Mizukoshi K, Takeuchi M, Nagata Y et al (2016) Normal values of left ventricular Mass Index assessed by Transthoracic three-Dimensional Echocardiography. J Am Soc Echocardiogr 29:51–61
Zheng X, Ran H, Ren J et al (2023) Two-dimensional speckle tracking imaging analyses of the correlations between left atrial appendage function and stroke risk in nonvalvular atrial fibrillation patients. Int J Cardiovasc Imaging Published Online December 1
Pluta A, Stróżecki P, Krintus M et al (2015) Left ventricular remodeling and arterial remodeling in patients with chronic kidney disease stage 1–3. Ren Fail 37:1105–1110
Gupta J, Dominic EA, Fink JC et al (2015) Association between inflammation and cardiac geometry in chronic kidney disease: findings from the CRIC Study. PLoS On 10:e0124772
Hassan MO, Duarte R, Dix-Peek T et al (2016) Correlation between volume overload, chronic inflammation, and left ventricular dysfunction in chronic kidney disease patients. Clin Nephrol 86:131–135
Demetgul H, Giray D, Delibas A et al (2018) 2D-Speckle tracking echocardiography contributes to early identification of impaired left ventricular myocardial function in children with chronic kidney disease. Cardiol Youn 28:1404–1409
Lee Y, Park S, Lee S et al (2020) Lipid profiles and risk of major adverse cardiovascular events in CKD and diabetes: a nationwide population-based study[J]. PLoS ONE 15:e0231328
Gan GCH, Bhat A, Chen HHL et al (2021) Left Atrial Reservoir strain by Speckle Tracking Echocardiography: Association with Exercise Capacity in chronic kidney disease. J Am Heart Asso 10:e017840
Jia G, Aroor AR, Hill MA et al (2018) Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension 72:537–548
Longobardo L, Suma V, Jain R et al (2017) Role of two-dimensional speckle-tracking echocardiography strain in the assessment of right ventricular systolic function and comparison with conventional parameters. J Am Soc Echocardiogr 30:937–946
Ohashi N, Isobe S, Ishigaki S et al (2019) Increased heart rate is associated with intrarenal renin-angiotensin system activation in chronic kidney disease patients. Clin Exp Nephrol 23:1109–1118
Cao W, Li A, Wang L et al (2015) A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol 26:1619–1633
Nohara A (2016) Epicardial adipose tissue as a predictor of plaque vulnerability in patients with mild chronic kidney disease. Circ J 80:64–66
Chen YC, Lee WH, Lee MK et al (2020) Epicardial adipose tissue thickness is not associated with adverse cardiovascular events in patients undergoing haemodialysis. Sci Rep 10:6281
Acknowledgements
We thank all the clinical staff for their support of this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Y.L drafting the article C.T the conception and design of the study Y. L, Y. L, and L.G: Acquisition of data, or analysis and interpretation of dataY. Li drafting the article C. T revising it critically for important intellectual content, and final approval of the version to be submitted.
Corresponding author
Ethics declarations
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, Y., Liu, Y., Gao, L. et al. Early impact of albuminuria on cardiac function in patients with chronic kidney disease: a prospective study. Int J Cardiovasc Imaging 40, 873–885 (2024). https://doi.org/10.1007/s10554-024-03056-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-024-03056-4