Abstract
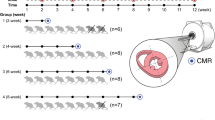
The characterization of cardiac mechanical properties may contribute to better understanding of doxorubicin-induced cardiotoxicity. Our study aims to investigate the relationship between cardiac mechanical properties, T1 and T2 relaxation times and partition coefficient. Fifty childhood acute lymphoblastic leukemia survivors underwent a cardiac magnetic resonance (CMR) at rest on a 3T MRI system and included a standard ECG-gated 3(3)3(3)5 MOLLI sequence for T1 mapping and an ECG-gated T2-prepared TrueFISP sequence for T2 mapping. Partition coefficient, ejection fraction, end-diastolic volume (EDV) and end-systolic volume (ESV) were calculated. CircAdapt model was used to study cardiac mechanical performance (left ventricle stiffness (LVS), contractility (LVC) and pressure (Pmin and Pmax), cardiac work efficiency (CWE) and ventricular arterial coupling). In the whole cohort, our results showed that LVC (R2 = 69.2%, r = 0.83), Pmin (R2 = 62.9%, r = 0.79) and Pmax can be predicted by significant CMR parameters, while T1 (R2 = 23.2%, r = 0.48) and partition coefficient (R2 = 13.8%, r = 0.37) can be predicted by significant cardiac mechanical properties. In SR group LVS (R2 = 94.8%, r = 0.97), LVC (R2 = 93.7%, r = 0.96) and Pmin (R2 = 90.6%, r = 0.95) can be predicted by significant cardiac mechanical properties, while in HR + DEX group CWE (R2 = 49.8%, r = 0.70) can be predicted by significant cardiac mechanical properties. Partition coefficient (R2 = 72.6%, r = 0.85) can be predicted by significant CMR parameters in SR group. Early characterization of cardiac mechanical properties from CMR parameters has the potential to early detect doxorubicin-induced cardiotoxicity.


Similar content being viewed by others
Data availability
Our data are not deposited in publicly available repositories. However, the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Kakaje A, Alhalabi MM, Ghareeb A, Karam B, Mansour B, Zahra B et al (2020) Rates and trends of childhood acute lymphoblastic leukaemia: an epidemiology study. Sci Rep 10(1):6756
Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT et al (2013) Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the american heart association. Circulation 128(17):1927–1995
Bansal N, Amdani SM, Hutchins KK, Lipshultz SE (2018) Cardiovascular disease in survivors of childhood cancer. Curr Opin Pediatr 30(5):628–638
Kang Y, Assuncao BL, Denduluri S, McCurdy S, Luger S, Lefebvre B et al (2019) Symptomatic heart failure in acute leukemia patients treated with anthracyclines. JACC CardioOncol 1(2):208–217
Caru M, Corbin D, Perie D, Lemay V, Delfrate J, Drouin S et al (2019) Doxorubicin treatments induce significant changes on the cardiac autonomic nervous system in childhood acute lymphoblastic leukemia long-term survivors. Clin Res Cardiol 108(9):1000–1008
Armstrong GT, Liu Q, Yasui Y, Neglia JP, Leisenring W, Robison LL et al (2009) Late mortality among 5-year survivors of childhood cancer: a summary from the childhood cancer survivor study. J Clin Oncol 27(14):2328–2338
Bansal N, Adams MJ, Ganatra S, Colan SD, Aggarwal S, Steiner R et al (2019) Strategies to prevent anthracycline-induced cardiotoxicity in cancer survivors. Cardiooncology 5:18
Podyacheva EY, Kushnareva EA, Karpov AA, Toropova YG (2021) Analysis of models of doxorubicin-induced cardiomyopathy in rats and mice. A modern view from the perspective of the pathophysiologist and the clinician. Front Pharmacol 12:670479
Seraphim A, Westwood M, Bhuva AN, Crake T, Moon JC, Menezes LJ et al (2019) Advanced imaging modalities to monitor for cardiotoxicity. Curr Treat Options Oncol 20(9):73
Migrino RQ, Aggarwal D, Konorev E, Brahmbhatt T, Bright M, Kalyanaraman B (2008) Early detection of doxorubicin cardiomyopathy using two-dimensional strain echocardiography. Ultrasound Med Biol 34(2):208–214
Wassmuth R, Lentzsch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R et al (2001) Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J 141(6):1007–1013
Burrage MK, Ferreira VM (2020) The use of cardiovascular magnetic resonance as an early non-invasive biomarker for cardiotoxicity in cardio-oncology. Cardiovasc Diagn Ther 10(3):610–624
Aissiou M, Perie D, Cheriet F, Dahdah NS, Laverdiere C, Curnier D (2013) Imaging of early modification in cardiomyopathy: the doxorubicin-induced model. Int J Cardiovasc Imag 29:1459–1476
Jiji RS, Kramer CM, Salerno M (2012) Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol 19(2):377–388
Nguyen-Truong M, Wang Z (2018) Biomechanical properties and mechanobiology of cardiac ECM. Adv Exp Med Biol 1098:1–19
Avazmohammadi R, Soares JS, Li DS, Raut SS, Gorman RC, Sacks MS (2019) A contemporary look at biomechanical models of myocardium. Annu Rev Biomed Eng 21:417–442
Silverman LB, Stevenson KE, O’Brien JE, Asselin BL, Barr RD, Clavell L et al (2010) Long-term results of dana-farber cancer institute ALL consortium protocols for children with newly diagnosed acute lymphoblastic (1985–2000). Leukemia 24(2):320–334
Marcoux S, Drouin S, Laverdiere C, Alos N, Andelfinger GU, Bertout L et al (2017) The PETALE study: late adverse effects and biomarkers in childhood acute lymphoblastic leukemia survivors. Pediatr Blood Cancer 64(6):1–8
Deichmann R, Haase A (1992) Quantification of T 1 values by SNAPSHOT-FLASH NMR imaging. J Magn Reson (1969) 96(3):608–612
Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV et al (2009) T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson: Off J Soc Cardiovasc Magn Reson 11:56
Li B, Liu Y, Occleshaw CJ, Cowan BR, Young AA (2010) In-line automated tracking for ventricular function with magnetic resonance imaging. JACC Cardiovasc Imag 3(8):860–866
Tsadok Y, Petrank Y, Sarvari S, Edvardsen T, Adam D (2013) Automatic segmentation of cardiac MRI cines validated for long axis views. Comput Med Imag Graph 37(7–8):500–511
Peng P, Lekadir K, Gooya A, Shao L, Petersen SE, Frangi AF (2016) A review of heart chamber segmentation for structural and functional analysis using cardiac magnetic resonance imaging. MAGMA 29(2):155–195
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance – 2020 update: Society for Cardiovascular magnetic resonance (SCMR): Board of Trustees Task Force on standardized post-processing. J Cardiovasc Magn Reson 22(1):19
Arts T, Delhaas T, Bovendeerd P, Verbeek X, Prinzen FW (2005) Adaptation to mechanical load determines shape and properties of heart and circulation: the CircAdapt model. Am J Physiol Heart Circ Physiol 288(4):H1943–H1954
Lumens J, Delhaas T (2012) Cardiovascular modeling in pulmonary arterial hypertension: focus on mechanisms and treatment of right heart failure using the CircAdapt model. Am J Cardiol 110(6 Suppl):39S–48S
Hau EM, Caccia JN, Kasteler R, Spycher B, Suter T, Ammann RA et al (2019) Cardiovascular disease after childhood acute lymphoblastic leukaemia: a cohort study. Swiss Med Wkly 149:w20012
James SL, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N et al (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. The Lancet 392(10159):1789–1858
Burkhoff D, Mirsky I, Suga H (2005) Assessment of systolic and diastolic ventricular properties via pressure-volume analysis: a guide for clinical, translational, and basic researchers. Am J Physiol Heart Circ Physiol 289(2):H501–H512
Shoucri RM (2011) Calculation of parameters of end-systolic pressure–volume relation in the ventricles. Math Comput Model 54(7–8):1638–1643
Lee SF, Luque-Fernandez MA, Chen YH, Catalano PJ, Chiang CL, Wan EY et al (2020) Doxorubicin and subsequent risk of cardiovascular diseases among survivors of diffuse large B-cell lymphoma in Hong Kong. Blood Adv 4(20):5107–5117
Faber J, Wingerter A, Neu MA, Henninger N, Eckerle S, Münzel T et al (2018) Burden of cardiovascular risk factors and cardiovascular disease in childhood cancer survivors: data from the german CVSS-study. Eur Heart J 39(17):1555–1562
Ellims AH, Shaw JA, Stub D, Iles LM, Hare JL, Slavin GS et al (2014) Diffuse myocardial fibrosis evaluated by post-contrast t1 mapping correlates with left ventricular stiffness. J Am Coll Cardiol 63(11):1112–1118
Salerno M, Janardhanan R, Jiji RS, Brooks J, Adenaw N, Mehta B et al (2013) Comparison of methods for determining the partition coefficient of gadolinium in the myocardium using T1 mapping. J Magn Reson Imag 38(1):217–224
Hendriks T, Said MA, Janssen LMA, van der Ende MY, van Veldhuisen DJ, Verweij N et al (2019) Effect of systolic blood pressure on left ventricular structure and function: a mendelian randomization study. Hypertension 74(4):826–832
Katz AM (2003) Heart failure: a hemodynamic disorder complicated by maladaptive proliferative responses. J Cell Mol Med 7(1):1–10
Aissiou M, Curnier D, Caru M, Hafyane T, Leleu L, Krajinovic M et al (2021) Detection of doxorubicin-induced cardiotoxicity using myocardial T1 and T2 relaxation times in childhood acute lymphoblastic leukemia survivors. Int J Cardiovasc Imag 38:873–882
Périé D, Dahdah N, Foudis A, Curnier D (2013) Multi-parametric MRI as an indirect evaluation tool of the mechanical properties of in-vitro cardiac tissues. BMC Cardiovasc Disord 13:24
Funding
This work was supported by the Institute of Cancer Research (ICR) of the Canadian Institutes of Health Research (CIHR), in collaboration with C17 Council, Canadian Cancer Society (CCS), Cancer Research Society (CRS), Garron Family Cancer Centre at the Hospital for Sick Children, Ontario Institute for Cancer Research (OICR) and Pediatric Oncology Group of Ontario (POGO). We also thank the Natural Sciences and Engineering Research Council of Canada (NSERC) and Polytechnique Montreal for the financial support, as well as researchers from the PETALE study for the opportunity to perform these complementary analyses in the childhood ALL survivor’s cohort. This research was also supported in part by MSc, PhD and postdoctoral study grants from the Canadian Research Data Centre Network and the Quebec inter-University Centre for Social Statistics, Cole Foundation, Fonds de Recherche du Québec – Santé (FRQS), Fonds de recherche du Québec - Nature et technologies (FRQNT), Natural Sciences and Engineering Research Council of Canada (NSERC), program MÉDITIS, Sainte-Justine University Hospital Center Foundation and Foundation of Stars, and the TransMedTech Excellence Postdoctoral Fellowship from the Canada First Research Excellence Fund through the TransMedTech Institute. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
MC, DC, GA, MK, CL, DS and DP conceived the study. EU, MC, MA performed data analysis and EU wrote the manuscript. All authors contributed to the experimental design and all authors revised the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Uwase, E., Caru, M., Curnier, D. et al. Relationship between cardiac mechanical properties and cardiac magnetic resonance imaging at rest in childhood acute lymphoblastic leukemia survivors. Int J Cardiovasc Imaging 39, 2589–2598 (2023). https://doi.org/10.1007/s10554-023-02953-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-023-02953-4