Abstract
Purpose
This study evaluated whether optical frequency domain imaging (OFDI) accurately distinguish between fibroatheroma (FA) and pathological intimal thickening (PIT) compared with histopathology.
Methods
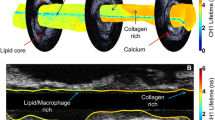
A total of 631 histological cross-sections from 14 autopsy hearts were analyzed for the comparison between OFDI and histological images. Of those, 190 (30%) sections were diagnosed with PIT and 120 (19%) with FA. The OFDI signal attenuation rate was calculated from an exponential. The lipid length was measured longitudinally by detection of sequential OFDI frames within a plaque segment containing lipids. The lipid arc was measured with a protractor centered in the center of the lumen. The fibrous cap thickness was defined as the minimum thickness of the signal rich band overlying PIT and FA.
Results
There was no significant difference in the OFDI signal attenuation rate between FA and PIT (3.09 ± 1.04 versus 2.79 ± 1.20, p = 0.13). However, the lipid length was significantly longer, the maximum lipid arc was significantly larger, and the fibrous cap thickness was significantly thinner in FA than in PIT (7.5 [4.3–10.3] mm versus 4.3 [2.7–5.8] mm, p < 0.0001, 125 [101–174]° versus 96 [74–131]°, p < 0.0001, and 220 [167–280] µm versus 260 [190–332] µm, p = 0.019).
Conclusions
This study revealed OFDI may have the potential capability for discriminating FA from PIT based on the longitudinal and circumferential extent of lipid plaque, although the OFDI signal attenuation rate was similar between FA and PIT.





Similar content being viewed by others
References
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20:1262–1275
Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M et al (2016) Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 13:79–98
Stary HC, Blankenhorn DH, Chandler AB, Glagov S, Insull W, Richardson M et al (1992) A definition of the intima of human arteries and of its atherosclerosis-prone regions. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 85:391–405
Brezinski ME, Tearney GJ, Bouma BE, Izatt JA, Hee MR, Swanson EA et al (1996) Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology. Circulation 93:1206–1213
Yabushita H, Bouma BE, Houser SL, Aretz HT, Jang IK, Schlendorf KH et al (2002) Characterization of human atherosclerosis by optical coherence tomography. Circulation 106:1640–1645
Kawasaki M, Takatsu H, Noda T, Ito Y, Kunishima A, Arai M et al (2001) Noninvasive quantitative tissue characterization and two-dimensional color-coded map of human atherosclerotic lesions using ultrasound integrated backscatter: comparison between histology and integrated backscatter images. J Am Coll Cardiol 38:486–492
Okubo M, Kawasaki M, Ishihara Y, Takeyama U, Kubota T, Yamaki T et al (2008) Development of integrated backscatter intravascular ultrasound for tissue characterization of coronary plaques. Ultrasound Med Biol 34:655–663
Fujii K, Kubo T, Otake H, Nakazawa G, Sonoda S, Hibi K et al (2020) Expert consensus statement for quantitative measurement and morphological assessment of optical coherence tomography. Cardiovasc Interv Ther 35:13–18
Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG et al (2012) Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol 59:1058–1072
Jang IK, Bouma BE, Kang DH, Park SJ, Park SW, Seung KB et al (2002) Visualization of coronary atherosclerotic plaques in patients using optical coherence tomography: comparison with intravascular ultrasound. J Am Coll Cardiol 39:604–609
Sonoda S, Hibi K, Okura H, Fujii K, Honda Y, Kobayashi Y (2020) Current clinical use of intravascular ultrasound imaging to guide percutaneous coronary interventions. Cardiovasc Interv Ther 35:30–36
Otsuka F, Joner M, Prati F, Virmani R, Narula J (2014) Clinical classification of plaque morphology in coronary disease. Nat Rev Cardiol 11:379–389
Allen TJ, Hall A, Dhillon AP, Owen JS, Beard PC (2012) Spectroscopic photoacoustic imaging of lipid-rich plaques in the human aorta in the 740 to 1400 nm wavelength range. J Biomed Opt 17:061209
Kume T, Akasaka T, Kawamoto T, Watanabe N, Toyota E, Neishi Y et al (2006) Assessment of coronary arterial plaque by optical coherence tomography. Am J Cardiol 97:1172–1175
van Soest G, Regar E, Goderie TP, Gonzalo N, Koljenović S, van Leenders GJ et al (2011) Pitfalls in plaque characterization by OCT: image artifacts in native coronary arteries. JACC Cardiovasc Imaging 4:810–813
Gnanadesigan M, Hussain AS, White S, Scoltock S, Baumbach A, van der Steen AF et al (2017) Optical coherence tomography attenuation imaging for lipid core detection: an ex-vivo validation study. Int J Cardiovasc Imaging 33:5–11
Kolodgie FD, Burke AP, Nakazawa G, Virmani R (2007) Is pathologic intimal thickening the key to understanding early plaque progression in human atherosclerotic disease? Arterioscler Thromb Vasc Biol 27:986–989
Ikenaga H, Ishihara M, Inoue I, Kawagoe T, Shimatani Y, Miura F et al (2013) Longitudinal extent of lipid pool assessed by optical coherence tomography predicts microvascular no-reflow after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. J Cardiol 62:71–76
Uzu K, Shinke T, Otake H, Takaya T, Osue T, Iwasaki M et al (2017) Morphological and pharmacological determinants of peri-procedural myocardial infarction following elective stent implantation: Optical coherence tomography sub-analysis of the PRASFIT-Elective study. J Cardiol 70:545–552
Fujii K, Hao H, Shibuya M, Imanaka T, Fukunaga M, Miki K et al (2015) Accuracy of OCT, grayscale IVUS, and their combination for the diagnosis of coronary TCFA: an ex vivo validation study. JACC Cardiovasc Imaging 8:451–460
Kotani J, Mintz GS, Castagna MT, Pinnow E, Berzingi CO, Bui AB et al (2003) Intravascular ultrasound analysis of infarct-related and non-infarct related arteries in patients who presented with an acute myocardial infarction. Circulation 107:2889–2893
Yamagishi M, Terashima M, Awano K, Kijima M, Nakatani S, Daikoku S et al (2000) Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol 35:106–111
Yamada R, Okura H, Kume T et al (2007) Histological characteristics of plaque with ultrasonic attenuation: a comparison between intravascular ultrasound and histology. J Cardiol 50:223–228
Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM et al (2018) Detection lipid core of coronary plaques in autopsy specimens with a novel cahterter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging 1:634–648
Goldstein J, Maini B, Dixon S, Brilakis E, Grines C, Rizik D et al (2011) Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circulation Cardiovasc Intervent 4:429–437
Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K et al (2015) Impact of Dual Lipid Lowering Strategy With Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients With Percutaneous Coronary Intervention: Insights From Multicenter Randomized Controlled PRECISE-IVUS Trial. J Am Coll Cardiol 66:495–507
Hibi K, Kozuma K, Sonoda S, Endo T, Tanaka H, Kyono H et al (2018) A Randomized Study of Distal Filter Protection Versus Conventional Treatment During Percutaneous Coronary Intervention in Patients With Attenuated Plaque Identified by Intravascular Ultrasound. JACC Cardiovasc Interv 11:1545–1555
Acknowledgements
The authors thank the staffs in the Department of Surgical Pathology at Hyogo College of Medicine for their excellent assistance for the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Takahiro Imanaka has nothing to declare for this study. Masaharu Ishihara received research fund from Amgen Inc. (Japan), and lecture fees from Bayer Yakuhin, Ltd. (Japan), Daiichi Sankyo Company (Japan), Nippon Boehringer Ingelheim Co., Ltd. (Japan), and Otsuka Pharmaceutical Co., Ltd. (Japan), and scholarship funds from Abbott Co.,Ltd. (Japan), Otsuka Pharmaceutical Co.,Ltd. (Japan), MID,Inc. (Japan), Ono Pharmaceutical Co.,Ltd. (Japan), Orbusneich Medical K. K. (Japan), Kowa Pharmaceutical Co.,Ltd. (Japan), Mitsubishi Tanabe Pharma Corporation (Japan), Terumo Corporation (Japan), Nipro Corporation (Japan), Lifeline Co.,Ltd. (Japan), Bayer Yakuhin, Ltd. (Japan), Fukuda Denshi Co.,Ltd. (Japan), and Boston Scientific K.K. (Japan), and endowed departments from Abbott (Japan), Medtronic Japan Co., Ltd. (Japan), and Nippon Boehringer Ingelheim Co., Ltd. (Japan). No other potential conflicts of interest relating to this article were reported.
Ethics approval
The study protocol was approved by the Institutional Review Board of Hyogo College of Medicine (approval number 3766).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imanaka, T., Fujii, K., Tanaka, T. et al. Potential of optical frequency domain imaging for differentiation between early and advanced coronary atherosclerosis. Int J Cardiovasc Imaging 38, 2791–2799 (2022). https://doi.org/10.1007/s10554-022-02600-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02600-4