Abstract
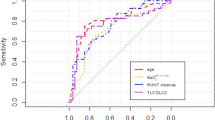
Previous reports suggested that poor pulmonary function was associated with increased arterial elastance (Ea) in patients with chronic obstructive pulmonary disease and systemic sclerosis. The mechanisms connecting pulmonary function and Ea have not yet been accurately studied in patients with idiopathic pulmonary fibrosis (IPF). The present study was designed to assess Ea in IPF patients without chronic severe pulmonary hypertension and to determine its prognostic role over a medium-term follow-up. This retrospective study included 60 consecutive patients with mild-to-moderate IPF (73.8 ± 6.6 years, 75% males) and 60 controls matched by age, sex and cardiovascular risk factors. All patients underwent physical examination, spirometry, blood tests, modified Haller index (MHI, chest transverse diameter over the distance between sternum and spine) assessment, conventional transthoracic echocardiography implemented with speckle tracking analysis of left atrial positive global strain (LA-GSA+ ) and finally carotid Doppler ultrasonography, at basal evaluation. The effective arterial elastance index (EaI) was calculated as the ratio of end-systolic pressure to stroke volume index. During follow-up period, we evaluated the composite endpoint of (1) pulmonary or cardiovascular hospitalizations; (2) all-cause mortality. At baseline, EaI was significantly higher in IPF patients than controls (4.1 ± 1.3 vs 3.5 ± 1.0 mmHg/ml/m2, p = 0.01). EaI was strongly correlated to the following variables: C-reactive protein (CRP) (r = 0.86), forced vital capacity (FVC) (r = − 0.91), E/e′ ratio (r = 0.91), LA-GSA+ (r = − 0.92), common carotid artery-cross sectional area (CCA-CSA) (r = 0.89) and MHI (r = 0.86), in IPF patients. Mean follow-up time was 2.4 ± 1.3 years. During follow-up, 12 patients died and 17 were hospitalized due to major adverse clinical events. At univariate Cox analysis, CRP (HR 1.51, 95% CI 1.25–1.82), FVC (HR 0.88, 95% CI 0.85–0.91), LA-GSA+ (HR 0.85, 95% CI 0.77–0.94), CCA-CSA (HR 1.12, 95% CI 1.03–1.22) and EaI (HR 2.43, 95% CI 1.75–3.37) were significantly associated with outcome. At multivariate Cox analysis, only EaI (HR 1.60, 95% CI 1.03–2.50) retained statistical significance. An EaI ≥ 4 mmHg/ml/m2 showed 100% sensitivity and 99.4% specificity for predicting outcome (AUC = 0.98). In patients with mild-to-moderate IPF, an EaI ≥ 4 mmHg/ml/m2 is a negative prognostic factor over a medium-term follow-up.




Similar content being viewed by others
Abbreviations
- 6MWT:
-
Six-Minute Walking Test
- AUC:
-
Area under curve
- CCA:
-
Common carotid artery
- CI:
-
Confidence interval
- COPD:
-
Chronic obstructive pulmonary disease
- CRP:
-
C-reactive protein
- CSA:
-
Cross sectional area
- DLCO:
-
Diffusing capacity of the lungs for carbon monoxide
- EaI:
-
Arterial elastance index
- EesI:
-
End-systolic elastance index
- EDD:
-
End-diastolic diameter
- eGFR:
-
Estimated glomerular filtration rate
- ESP:
-
End-systolic pressure
- FVC:
-
Forced vital capacity
- GSA+ :
-
Positive global atrial strain
- ICC:
-
Intraclass correlation coefficient
- ILD:
-
Interstitial lung disease
- IMT:
-
Intima–media thickness
- IPF:
-
Idiopathic pulmonary fibrosis
- LA:
-
Left atrial
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- LVESVi:
-
Left ventricular end-systolic volume index
- MACE:
-
Major adverse clinical events
- MHI:
-
Modified Haller index
- ROC:
-
Receiver operating characteristic
- RWT:
-
Relative wall thickness
- SPAP:
-
Systolic pulmonary artery pressure
- STE:
-
Speckle tracking echocardiography
- SVi:
-
Stroke volume index
- TRV:
-
Tricuspid regurgitation velocity
- VAC:
-
Ventricular-arterial coupling
References
Raghu G, Remy-Jardin M, Myers JL et al (2018) Diagnosis of idiopathic pulmonary fibrosis: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 198:e44–e68
Harari S, Davì M, Biffi A et al (2020) Epidemiology of idiopathic pulmonary fibrosis: a population-based study in primary care. Intern Emerg Med 15:437–445
Dalleywater W, Powell HA, Hubbard RB, Navaratnam V (2015) Risk factors for cardiovascular disease in people with idiopathic pulmonary fibrosis: a population-based study. Chest 147:150–215
Suzuki A, Kondoh Y (2017) The clinical impact of major comorbidities on idiopathic pulmonary fibrosis. Respir Investig 55:94–103
van Cleemput J, Sonaglioni A, Wuyts WA, Bengus M, Stauffer JL, Harari S (2019) Idiopathic pulmonary fibrosis for cardiologists: differential diagnosis, cardiovascular comorbidities, and patient management. Adv Ther 36:298–317
King TE Jr, Albera C, Bradford WZ et al (2014) All-cause mortality rate in patients with idiopathic pulmonary fibrosis. Implications for the design and execution of clinical trials. Am J Respir Crit Care Med 189:825–831
King CS, Nathan SD (2017) Idiopathic pulmonary fibrosis: effects and optimal management of comorbidities. Lancet Respir Med 5:72–84
Caminati A, Lonati C, Cassandro R et al (2019) Comorbidities in idiopathic pulmonary fibrosis: an underestimated issue. Eur Respir Rev 28:190044
Chemla D, Antony I, Lecarpentier Y, Nitenberg A (2003) Contribution of systemic vascular resistance and total arterial compliance to effective arterial elastance in humans. Am J Physiol Heart Circ Physiol 285:H614–H620
Antonini-Canterin F, Poli S, Vriz O et al (2013) The ventricular-arterial coupling: from basic pathophysiology to clinical application in the echocardiography laboratory. J Cardiovasc Echogr 23:91–95
Sunagawa K, Maughan WL, Burkhoff D, Sagawa K (1983) Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol 245(5 Pt 1):H773–H780
Chantler PD, Lakatta EG, Najjar SS (2008) Arterial-ventricular coupling: mechanistic insights into cardiovascular performance at rest and during exercise. J Appl Physiol 105:1342–1351
Willum-Hansen T, Staessen JA, Torp-Pedersen C et al (2006) Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation 113:664–670
Vlachopoulos C, Aznaouridis K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55:1318–1327
Mills NL, Miller JJ, Anand A et al (2008) Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax 63:306–311
Moyssakis I, Gialafos E, Vassiliou V et al (2005) Aortic stiffness in systemic sclerosis is increased independently of the extent of skin involvement. Rheumatology (Oxford) 44:251–254
Okamoto M, Shipley MJ, Wilkinson IB et al (2019) Does poorer pulmonary function accelerate arterial stiffening? A cohort study with repeated measurements of carotid-femoral pulse wave velocity. Hypertension 74:929–935
Alageel S, Gulliford MC (2019) Health checks and cardiovascular risk factor values over six years’ follow-up: Matched cohort study using electronic health records in England. PLoS Med 16:e1002863
Sonaglioni A, Caminati A, Lipsi R et al (2020) Early left atrial dysfunction in idiopathic pulmonary fibrosis patients without chronic right heart failure. Int J Cardiovasc Imaging 36:1711–1723
Sonaglioni A, Caminati A, Lipsi R, Lombardo M, Harari S (2021) Association between C-reactive protein and carotid plaque in mild-to-moderate idiopathic pulmonary fibrosis. Intern Emerg Med 16:1529–1539
Harari S, Cereda F, Pane F et al (2019) Lung cryobiopsy for the diagnosis of interstitial lung diseases: a series contribution to a debated procedure. Medicina (Kaunas) 55:606
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130:461–470
Sonaglioni A, Baravelli M, Vincenti A et al (2018) A new modified anthropometric Haller index obtained without radiological exposure. Int J Cardiovasc Imaging 34:1505–1509
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 28:1-39.e14
Nagueh SF, Smiseth OA, Appleton CP et al (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 29:277–314
Rudski LG, Lai WW, Afilalo J et al (2010) Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 23:685–713
Badano LP, Kolias TJ, Muraru D et al (2018) Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging 19:591–600
Sonaglioni A, Lonati C, Lombardo M et al (2019) Incremental prognostic value of global left atrial peak strain in women with new-onset gestational hypertension. J Hypertens 37:1668–1675
Stein JH, Korcarz CE, Hurst RT et al (2008) Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force: endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 21:93–111
AIUM (2016) AIUM practice parameter for the performance of an ultrasound examination of the extracranial cerebrovascular system. J Ultrasound Med 35:1–11
Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA (2005) Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation 112:2254–2262
van Popele NM, Grobbee DE, Bots ML et al (2001) Association between arterial stiffness and atherosclerosis: the Rotterdam study. Stroke 32:454–460
Palombo C, Kozakova M (2016) Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vasc Pharmacol 77:1–7
Yasmin MCM, Wallace S et al (2005) Matrix metalloproteinase-9 (MMP-9), MMP-2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 25:372
Bolton CE, Cockcroft JR, Sabit R et al (2009) Lung function in mid-life compared with later life is a stronger predictor of arterial stiffness in men: the caerphilly prospective study. Int J Epidemiol 38:867–876
Amaral AF, Patel J, Gnatiuc L, Jones M, Burney PG (2015) Association of pulse wave velocity with total lung capacity: a cross-sectional analysis of the BOLD London study. Respir Med 109:1569–1575
Eagan TM, Ueland T, Wagner PD et al (2010) Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J 35:540–548
Agusti A, Edwards LD, Rennard SI et al (2012) Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS ONE 7:e37483
Ferns GAA, Heikal L (2017) Hypoxia in atherogenesis. Angiology 68:472–493
Tarbell J, Mahmoud M, Corti A, Cardoso L, Caro C (2020) The role of oxygen transport in atherosclerosis and vascular disease. J R Soc Interface 17:20190732
Wen W, Luo R, Tang X et al (2015) Age-related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis 238:147–152
Tesauro M, Mauriello A, Rovella V et al (2017) Arterial ageing: from endothelial dysfunction to vascular calcification. J Intern Med 281:471–482
Mikael LR, Paiva AMG, Gomes MM et al (2017) Vascular aging and arterial stiffness. Arq Bras Cardiol 109:253–258
Randrianarisoa E, Rietig R, Jacob S et al (2015) Normal values for intima-media thickness of the common carotid artery: an update following a novel risk factor profiling. Vasa 44:444–450
Archer JE, Gardner A, Berryman F, Pynsent P (2016) The measurement of the normal thorax using the Haller index methodology at multiple vertebral levels. J Anat 229:577–581
Sonaglioni A, Nicolosi GL, Granato A, Lombardo M, Anzà C, Ambrosio G (2021) Reduced myocardial strain parameters in subjects with pectus excavatum: impaired myocardial function or methodological limitations due to chest deformity? Semin Thorac Cardiovasc Surg 33:251–262
Sonaglioni A, Rigamonti E, Nicolosi GL, Bianchi S, Lombardo M (2021) Influence of chest conformation on ventricular-arterial coupling during normal pregnancy. J Clin Ultrasound 49:586–596
Chen CH, Fetics B, Nevo E et al (2001) Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38:2028–2034
Chantler PD, Lakatta EG (2012) Arterial-ventricular coupling with aging and disease. Front Physiol 3:90
Satoh H, Kurishima K, Ishikawa H, Ohtsuka M (2006) Increased levels of KL-6 and subsequent mortality in patients with interstitial lung diseases. J Intern Med 260:429–434
Kinder BW, Brown KK, McCormack FX et al (2009) Serum surfactant protein-A is a strong predictor of early mortality in idiopathic pulmonary fibrosis. Chest 135:1557–1563
Barlo NP, van Moorsel CH, Ruven HJ, Zanen P, van den Bosch JM, Grutters JC (2009) Surfactant protein-D predicts survival in patients with idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 26:155–161
Rosas IO, Richards TJ, Konishi K et al (2008) MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med 5:e93
Funding
This work has been supported by Italian Ministry of Health Ricerca Corrente—IRCCS MultiMedica.
Author information
Authors and Affiliations
Contributions
AS: Conceptualization; Data curation; Investigation; Methodology; Software; Visualization; Writing—original draft. AC: Conceptualization; Data curation; Methodology; Writing—review & editing. GLN: Conceptualization; Supervision; Validation; Writing—review & editing. ML: Conceptualization; Supervision; Validation; Writing—review & editing. SH: Conceptualization; Supervision; Validation; Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
We wish to confirm that there are no conflicts of interest associated with this publication. Andrea Sonaglioni declares that he has no conflict of interest. Antonella Caminati reports personal fees from Roche and Boehringer Ingelheim, outside the submitted work. Gian Luigi Nicolosi declares that he has no conflict of interest. Michele Lombardo declares that he has no conflict of interest. Sergio Harari reports grants and personal fees from Roche, Actelion and Boehringer Ingelheim, outside the submitted work.
Human and animal rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
The need for informed consent was not required due to the retrospective nature of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10554_2022_2541_MOESM1_ESM.docx
Supplemental table. Intra- and interobserver variability analysis of the main echoDoppler parameters and hemodynamic indices. ICC, intraclass correlation coefficient. CCA, common carotid artery. CI, confidence interval. CSA, cross sectional area. EaI, arterial elastance index. GSA+, positive global atrial strain. LA, left atrial (DOCX 21 KB)
Rights and permissions
About this article
Cite this article
Sonaglioni, A., Caminati, A., Nicolosi, G.L. et al. Incremental prognostic value of arterial elastance in mild-to-moderate idiopathic pulmonary fibrosis. Int J Cardiovasc Imaging 38, 1473–1485 (2022). https://doi.org/10.1007/s10554-022-02541-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-022-02541-y