Abstract
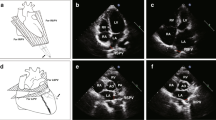
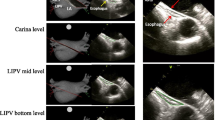
Preliminary data in human suggest that both Intracardiac echocardiography (ICE) and Intravascular ultrasound (IVUS) can be used for real-time information on the left atrial (LA) wall thickness and on the acute tissue changes produced by energy delivery. This pilot study was conducted to compare ICE and IVUS for real-time LA wall imaging and assessment of acute tissue changes produced by radiofrequency (RF), cryo and laser catheter ablation. Patients scheduled for RF, cryoballoon or laser balloon Pulmonary Vein Isolation (PVI) catheter ablation were enrolled. Each pulmonary vein (PV) was imaged before and immediately after ablation with either ICE or IVUS. The performance of ICE and IVUS for imaging were compared. Pre- and post-ablation measurements (lumen and vessel diameters, areas and sphericity indexes, wall thickness and muscular sleeve thickness) were taken at the level of each PV ostium. A total of 48 PVs in 12 patients were imaged before and after ablation. Both ICE and IVUS showed acute tissue changes. Compared to IVUS, ICE showed higher imaging quality and inter-observer reproducibility of the PV measurements obtained. Acute wall thickening suggestive of oedema was observed after RF treatment (p = 0.003) and laser treatment (p = 0.003) but not after cryoablation (p = 0.69). Our pilot study suggests that ICE might be preferable to IVUS for LA wall thickness imaging at the LA-PV junctions during ablation. Ablation causes acute wall thickening when using RF or laser energy, but not cryoenergy delivery. Larger studies are needed to confirm these preliminary findings.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- AF:
-
Atrial fibrillation
- ICE:
-
Intracardiac echocardiography
- IQR:
-
Interquartile range
- IVUS:
-
Intravascular ultrasound
- LA:
-
Left atrial
- PV:
-
Pulmonary vein
- PVI:
-
Pulmonary vein isolation
- PVs:
-
Pulmonary veins
- RF:
-
Radiofrequency
- SD:
-
Standard deviation
- WTI:
-
Wall thickness index
- WTI%:
-
Wall thickness index percentage
References
Hall B, Jeevanantham V, Simon R, Filippone J, Vorobiof G, Daubert J (2006) Variation in left atrial transmural wall thickness at sites commonly targeted for ablation of atrial fibrillation. J Interv Card Electrophysiol 17:127–132
Ho SY, Sanchez-Quintana D, Cabrera JA, Anderson RH (1999) Anatomy of the left atrium: implications for radiofrequency ablation of atrial fibrillation. J Cardiovasc Electrophysiol 10:1525–1533
Schwartzman D, Ren JF, Devine WA, Callans DJ (2001) Cardiac swelling associated with linear radiofrequency ablation in the atrium. J Interv Card Electrophysiol 5:159–166
Weerasooriya R, Jaïs P, Sanders P et al (2003) Images in cardiovascular medicine. Early appearance of an edematous tissue reaction during left atrial linear ablation using intracardiac echo imaging. Circulation 108:e80
Okada T, Yamada T, Murakami Y et al (2007) Prevalence and severity of left atrial edema detected by electron beam tomography early after pulmonary vein ablation. J Am Coll Cardiol 49:1436–1442
Yamada T, Murakami Y, Okada T et al (2006) Incidence, location, and cause of recovery of electrical connections between the pulmonary veins and the left atrium after pulmonary vein isolation. Europace: Eur Pacing Arrhythm Card Electrophysiol: J Work Groups Card Pacing arrhythm Card Cell Electrophysiol Eur Soc Cardiol 8:182–188
Arujuna A, Karim R, Caulfield D et al (2012) Acute pulmonary vein isolation is achieved by a combination of reversible and irreversible atrial injury after catheter ablation: evidence from magnetic resonance imaging. Circ Arrhythm Electrophysiol 5:691–700
Ren JF, Callans DJ, Schwartzman D, Michele JJ, Marchlinski FE (2001) Changes in local wall thickness correlate with pathologic lesion size following radiofrequency catheter ablation: an intracardiac echocardiographic imaging study. Echocardiography 18:503–507
Granier M, Winum PF, Granier M et al (2015) Real-time atrial wall imaging during radiofrequency ablation in a porcine model. Heart Rhythm 12:1827–1835
Baran J, Lewandowski P, Smarż K, Sikorska A, Zaborska B, Kułakowski P (2017) Acute hemodynamic and tissue effects of cryoballoon ablation on pulmonary vessels: the IVUS-Cryo study. J Am Heart Assoc 6(6):e005988
Motoike Y, Harada M, Ito T et al (2021) Wall thickness-based adjustment of ablation index improves efficacy of pulmonary vein isolation in atrial fibrillation: real-time assessment by intracardiac echocardiography. J Cardiovasc Electrophysiol 32:1620–1630
Gao XA-O, Chang DA-O, Bilchick KC et al (2021) Left atrial thickness and acute thermal injury in patients undergoing ablation for atrial fibrillation: laser versus radiofrequency energies. J Cardiovasc Electrophysiol 32:1259–1267
Mintz GS, Nissen SE, Anderson WD et al (2001) American college of cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (IVUS). A report of the American College of Cardiology Task Force on clinical expert consensus documents. J Am Coll Cardiol 37:1478–1492
Cabrera JA, Sánchez-Quintana D, Farré J et al (2002) Ultrasonic characterization of the pulmonary venous wall: echographic and histological correlation. Circulation 106:968–973
Ang R, Hunter RJ, Baker V et al (2015) Pulmonary vein measurements on pre-procedural CT/MR imaging can predict difficult pulmonary vein isolation and phrenic nerve injury during cryoballoon ablation for paroxysmal atrial fibrillation. Int J Cardiol 195:253–258
Jongbloed MR, Bax JJ, Lamb HJ et al (2005) Multislice computed tomography versus intracardiac echocardiography to evaluate the pulmonary veins before radiofrequency catheter ablation of atrial fibrillation: a head-to-head comparison. J Am Coll Cardiol 45:343–350
Wittkampf FH, Vonken EJ, Derksen R et al (2003) Pulmonary vein ostium geometry: analysis by magnetic resonance angiography. Circulation 107:21–23
Haines DE, Wright M, Harks E et al (2017) Near-field ultrasound imaging during radiofrequency catheter ablation: tissue thickness and epicardial wall visualization and assessment of radiofrequency ablation lesion formation and depth. Circ Arrhythm Electrophysiol 10:e005295
Wright M, Harks E, Deladi S et al (2018) Characteristics of radiofrequency catheter ablation lesion formation in real time in vivo using near field ultrasound imaging. JACC Clin Electrophysiol 4:1062–1072
Reddy VY, Neuzil P, Themistoclakis S et al (2009) Visually-guided balloon catheter ablation of atrial fibrillation: experimental feasibility and first-in-human multicenter clinical outcome. Circulation 120:12–20
Gerstenfeld EP, Michele J (2010) Pulmonary vein isolation using a compliant endoscopic laser balloon ablation system in a swine model. J Interv Card Electrophysiol 29:1–9
Khairy P, Dubuc M (2008) Transcatheter cryoablation part I: preclinical experience. Pacing Clin Electrophysiol: PACE 31:112–120
Lustgarten DL, Keane D, Ruskin J (1999) Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog Cardiovasc Dis 41:481–498
Gage AA, Baust J (1998) Mechanisms of tissue injury in cryosurgery. Cryobiology 37:171–186
de Bakker JM, Ho SY, Hocini M (2002) Basic and clinical electrophysiology of pulmonary vein ectopy. Cardiovasc Res 54:287–294
Acknowledgements
We thank the John Radcliffe Hospital Cardiac Angiography Suite Team and the Oxford Cardiac Research Team for their invaluable help and support for the conduction of the study.
Funding
This study was funded by a grant of the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre. Prof. Betts is supported by the Oxford NIHR Biomedical Research Centre. Dr Leo’s salary was supported by an unrestricted research grant from Abbott Medical.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ML and GLDM. The first draft of the manuscript was written by ML and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Dr Ginks has received speaker’s fees from Abbott and Biosense Webster. Dr Rajappan has received speaker’s fees from Abbott. Dr Hunter has received research grants, educational grants and speaker’s fees from Biosense Webster and Medtronic; he is an inventor of the STAR Mapping system and shareholder in Rhythm AI Ltd. Dr Betts has received research funding, speaker’s fees and advisory board fees from Abbott. The other authors have no conflict of interests to declare.
Ethical approval
The INSIDE PVs study was approved by the Health Research Authority West Midlands Coventry and Warwickshire Research Ethics committee and conducted in accordance with the Declaration of Helsinki (REC number 16/WM/0379).
Informed consent
All patients provided informed consent for participation in the INSIDE PVs study. All patients provided informed consent for anonymous publication of the results of the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Leo, M., De Maria, G.L., Briosa e Gala, A. et al. INtra-procedural ultraSound Imaging for DEtermination of atrial wall thickness and acute tissue changes after isolation of the pulmonary veins with radiofrequency, cryoballoon or laser balloon energy: the INSIDE PVs study. Int J Cardiovasc Imaging 37, 3525–3535 (2021). https://doi.org/10.1007/s10554-021-02417-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-021-02417-7