Abstract
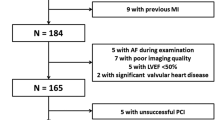
No-reflow (NR) is one of the major complications of primary percutaneous coronary intervention (PCI) in patients with non-ST-segment elevation myocardial infarction (NSTEMI). We aim to assess the value of multilayer longitudinal strain parameter to predict NR in patients with NSTEMI and preserved ejection fraction. 230 consecutive patients who were admitted to the emergency department and diagnosed with NSTEMI were prospectively included in this study. Echocardiography was performed 1 h before angiography. Specific analysis for endocardial, mid-myocardial and epicardial layers were performed by two-dimensional (2D) speckle tracking echocardiography (STE) for multilayer longitudinal strain. NR was described as flow grade of ≤ TIMI 2 when mechanical occlusions like dissection, intimal tear, arterial spasm and thromboembolism during angiography were excluded. 49 of 168 patients admitted to the study had NR. No significant differences were observed between the groups regarding age and gender. Multilayer longitudinal strain imaging (endocard, midmyocard and epicard) revealed lower strain values particularly in endocardial layer in patients with NR (GLS-endocard: − 14.14 ± 1.39/− 17.41 ± 2.34, p < 0.001; GLS-midmyocard: − 14.81 ± 1.40/17.81 ± 2.22, p < 0.001; GLS-epicard: − 16.14 ± 1.38/18.22 ± 2.00, p < 0.001). GLS-endocard, GLS-midmyocard, GLS-epicard and ST depression were found to be statistically significant independents parameters respectively to predict NR phenomenon (GLS-endocard: OR: 2.193, p < 0.001; GLS-midmyocard: OR: 1.510, p: 0.016; GLS-epicard: OR: 1.372, p: 0.035; ST depression: OR: 3.694, p: 0.014). We revealed that left ventricular strain study with speckle tracking echocardiography predicts NR formation. This noninvasive method may be useful for detecting NR formation in patients with NSTEMI.



Similar content being viewed by others
References
Rezkalla SH, Kloner RA (2008) Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 72:950–957
Beaglehole R, Irwin A, Prentice T (2004) The World Health Report 2004: changing history. World Health Organization, Geneva
Ito H, Tomooka T, Sakai N et al (1992) Lack ofmyocardial perfusion immediately aftersuccessful thrombolysis: a predictor of poor recovery of left ventricularfunction in anterior myocardial infarction. Circulation 85:1699–1705
Ito H, Maruyama A, Iwakura K et al (1996) Clinical implications of the ‘no reflow’phenomenon: a predictor of complications and left ventricular remodelling inreperfused anterior wall myocardial infarction. Circulation 93:223–228
Ito H, Iwakura K (1998) Assessing the relation between coronary reflow andmyocardial reflow. Am J Cardiol 81(Suppl. 12A):8G–12G
Morishima I, Sone T, Mokuno S et al (1995) Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J 130:239–243
Morishima I, Sone T, Okumura K et al (2000) Angiographic no-reflow phenomenon as a predictorof adverse longterm outcome in patients treated with percutaneous transluminal coronaryangioplasty for first acute myocardial infarction. J Am Coll Cardiol 36:1202–1209
Marzilli M, Gliozheni E, Marraccini P, Fedele S (1998) Primary coronary angioplasty in acute myocardial infarction: clinical correlates of the 'no reflow' phenomenon. Int J Cardiol 65(Suppl 1):S23–S28
Dahlslett T, Karlsen S, Grenne B et al (2014) Early assessment of strain echocardiography can accurately exclude significant coronary artery stenosis in suspected non-ST-segment elevation acute coronary syndrome. J Am Soc Echocardiogr 27:512–519
Eek C, Grenne B, Brunvand H et al (2010) Strain echocardiography predicts acute coronary occlusion in patientswith non-ST-segment elevation acute coronary syndrome. Eur J Echocardiogr 11:501–508
Grenne B, Eek C, Sjøli B et al (2010) Acute coronary occlusion in non-ST-elevation acute coronary syndrome: outcome and early identification by strain echocardiography. Heart 96:1550–1556
Liang HY, Cauduro S, Pellikka P, Wang J, Urheim S, Yang EH et al (2006) Usefulness of two-dimensional speckle strain for evaluation of left ventricular diastolic deformation in patients with coronary artery disease. Am J Cardiol 98:1581–1586
Choi JO, Cho SW, Song YB, Cho SJ, Song BG, Lee SC et al (2009) Longitudinal 2D strain at rest predicts the presence of left main and three vessel coronary artery disease in patients without regional wall motion abnormality. Eur J Echocardiogr 10:695–701
Nucifora G, Schuijf JD, Delgado V, Bertini M, Scholte AJ, Ng AC et al (2010) Incremental value of subclinical left ventricular systolic dysfunction for the identification of patients with obstructive coronary artery disease. Am Heart J 159:148–157
Montgomery DE, Puthumana JJ, Fox JM, Ogunyankin KO (2012) Global longitudinal strain aids the detection of non-obstructive coronary artery disease in the resting echocardiogram. Eur Heart J Cardiovasc Imaging 13:579–587
Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M et al (2015) ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European society of cardiology (ESC). Eur Heart J 37(3):267–315
Scanlon PJ, Faxon DP, Audet AM et al (1999) ACC/AHA Guidelines for coronary angiography: executive summary and recommendations: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaborationwith the society for cardiac angiography and interventions. Circulation 99:2345–2357
Gibson CM, Cannon CP, Daley WL, Dodge JT Jr, Alexander B Jr, Marble SJ, McCabe CH, Raymond L, Fortin T, Poole WK (1996) TIMI frame count: a quantitative method of assessing coronary artery flow. Braunwald ECirc 93(5):879–888
Muller O, Trana C, Curr EE (2013) Myocardial no-reflow treatment. Vasc Pharmacol 11(2):278–285
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270
de Simone G (1992) Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 20(5):1251−1260
Assali AR, Sdringola S, Ghani M et al (2000) Intracoronary adenosine administered during percutaneous intervention in acute myocardial infarction and reduction in theincidence of "no reflow" phenomenon. Catheter Cardiovasc Interv 51:27–31
Vallejo E, Pena-Duque MA, Norono O et al (1998) The no-reflow phenomenon: its incidence andclinical characteristics in a series of cases. Arch Inst Cardiol Mex 68:247–252
Brosh D, Assali AR, Mager A et al (2007) Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-monthmortality. Am J Cardiol 99(4):442–445
Ndrepepa G, Tiroch K, Fusaro M et al (2010) 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol 55(21):2383–2389
Feher A, Chen SY, Bagi Z, Arora V (2014) Prevention and treatment of no-reflow phenomenon by targeting the coronary microcirculation. Rev Cardiovasc Med 15(1):38–51
Jaffe R, Charron T, Puley G, Dick A, Strauss BH (2008) Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation 117(24):3152–3156
Harrison RW et al (2013) Incidence and outcomes of no-reflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol 111(2):178–184. https://doi.org/10.1016/j.amjcard.2012.09.015
Bolognese L et al (2004) Impact of microvascular dysfunction on left ventricular remodelling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation 109:1121–1126
Yesin M, Çağdaş M, Kalçık M et al (2017) Comparison of syntax score andsyntax score II to predict “no reflow phenomenon” in patients with STsegmentelevation myocardial infarction. Int J Cardiovasc Imaging 17:1200–1205
Wang JW, Zhou ZQ, Chen YD et al (2015) A risk score for no-reflow in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Clin Cardiol 38:208–221
Leclercq F, Messner-Pellenc P, Descours Q et al (1999) Combined assessment of reflow and collateral blood flow by myocardial contrast echocardiography after acute reperfused myocardialinfarction. Heart 82:62–67
Santoro GM, Valenti R, Buonamici P et al (1998) Relation between ST-segment changes and myocardial perfusion evaluated by myocardial contrast echocardiography in patients with acutemyocardial infarction treated by direct angioplasty. Am J Cardiol 82:932–937
Schofer J, Montz R, Mathey D (1985) Scintigraphic evidence of the no-reflow phenomenon in human beings after coronary thrombolysis. J Am Coll Cardiol 5:593–598
Iwakura K, Hiroshi I, Shin T et al (1996) Alternation in the coronary blood flow velocity pattern in patients with no reflow and reperfused acute myocardial infarction. Circulation 94:1269–1275
Geyer H, Caracciolo G, Abe H et al (2010) Assesment of myocardial mechanics using speckle tracking echocardiography: fundaments and clinical applications. J Am Soc Echocardiogr 23:351–369
Horie H, Takahashi M, Minai K (1998) Long-term beneficial effect of late reperfusion for acute anterior myocardial infarction with percutaneous transluminal coronary angioplasty. Circulation 98:2377–2382
Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E et al (2006) Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47:789–793
Zhang L, Wu W-C, Ma H, Wang H (2016) Usefulness of layer-specific strain for identifying complex CAD and predicting the severity of coronary lesions in patients with non-ST-segment elevation acute coronary syndrome: compared with syntax score. Int J Cardiol 223:1045–1052
LeWinter MM et al (1975) Regional differences in myocardial performance in the left ventricle of the dog. Circ Res 37:191
Sabbah HN et al (1981) The relative role of subendocardium and subepicardium in left ventricular mechanics. Am J Physiol 240:H920
Kimura K et al (2011) Reproducibility and diagnostic accuracy of three-layer speckle tracking echocardiography in a swine chronic ischemia model. Echocardiography 28:1148
Ishizu T et al (2010) Experimental validation of left ventricular transmural strain gradient with echocardiographic two-dimensional speckle tracking imaging. Eur J Echocardiogr 11:377
Ono S et al (1995) Effect of coronary artery reperfusion on transmural myocardial remodeling in dogs. Circulation 91:1143
Wolferth CC, Bellet S, Livezey MM, Murphy F (1945) Negative displacement of the RS-T segment in the electrocardiogram and its relationships to positive displacement: an experimental study. Am Heart J 29:220–244
Bayley RH (1946) The electrocardiographic effects of injury at the endocardial surface of the left ventricle. Am Heart J 31:677–684
Katus HA, Looser S, Hallermayer K et al (1992) Development and in vitro characterization of a new immunoassay of cardiac troponin T. Clin Chem 38:386–393
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors are requested to disclose any actual or potential conflict of interest including any financial, personal or other relationships with other people or organizations within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, their work. If there are no conflicts of interest, the COI should read: “The authors report no relationships that could be construed as a conflict of interest”.
Rights and permissions
About this article
Cite this article
Atıcı, A., Barman, H.A., Erturk, E. et al. Multilayer longitudinal strain can help predict the development of no-reflow in patients with acute coronary syndrome without ST elevation. Int J Cardiovasc Imaging 35, 1811–1821 (2019). https://doi.org/10.1007/s10554-019-01623-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01623-8