Abstract
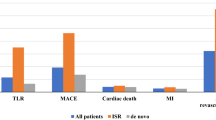
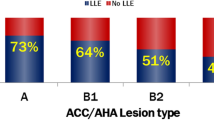
The purpose of this observational study was to investigate the feasibility, initial safety, and efficacy of the SeQuent® Please DCB (B. Braun Melsungen, Germany) for patients with de novo coronary lesions in vessels exceeding 3.0 mm in a consecutive series of all comer percutaneous coronary intervention. A total of 120 patients (135 lesions) with de novo coronary lesions in vessels ≥ 3.0 mm treated with DCB were enrolled in this single-centre prospective observational study. The primary endpoint was target lesion failure (TLF), a composite endpoint of cardiac death, target vessel-myocardial infarction (TV-MI), and clinically driven target vessel revascularization (TLR) at 12 months. Safety endpoints included cardiac death, TV-MI, and definite target vessel thrombosis. 45.9% of the lesions were classified as complex (type B2/C). The reference vessel diameter was 3.09 ± 0.31 mm measured via quantitative coronary angiography analysis. Coronary dissections occurred in 42 patients (35.0%; Type A-B 14.1%; Type C 19.1%; Type D: 1.6%), two of which [1.6%; (type D dissection)] underwent bail-out stent implantation. 12-month follow-up was completed in 100% patients. The 12-month incidence of TLF was 3.4%. The clinically driven TLR occurred in four patients (3.4%). The incidence of TLR was low in patients without any detectable dissections, similar to those with dissections (3.8% vs. 2.5%; p = 0.146). No patient suffered cardiac death, TV-MI, or target vessel thrombosis. The study shows the feasibility, initial safety, and efficacy of coronary intervention using SeQuent® Please DCB for the treatment of patients with de novo lesion in vessels exceeding 3 mm. The study highlights that the coronary dissection (Type A–C) post DCB treatment occurs frequently but is safe at follow up.



Similar content being viewed by others
References
Morice MC, Serruys PW, Sousa JE et al (2002) A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 346(23):1773–1780
Stone GW, Ellis SG, Cox DA et al (2004) A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med 350(3):221–231
Kereiakes DJ, Yeh RW, Massaro JM et al (2015) Stent thrombosis in drug-eluting or bare-metal stents in patients receiving dual antiplatelet therapy. JACC Cardiovasc Interv 8(12):1552–1562
Latib A, Colombo A, Castriota F et al (2012) A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels: the BELLO (Balloon Elution and Late Loss Optimization) study. J Am Coll Cardiol 60(24):2473–2480
Waksman R, Serra A, Loh JP et al (2013) Drug-coated balloons for de novo coronary lesions: results from the Valentines II trial. EuroIntervention 9(5):613–619
Nishiyama N, Komatsu T, Kuroyanagi T et al (2016) Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol 222:113–118
Miglionico M, Mangiacapra F, Nusca A et al (2015) Efficacy and safety of paclitaxel-coated balloon for the treatment of in-stent restenosis in high-risk patients. Am J Cardiol 116(11):1690–1694
López Mínguez JR, Nogales Asensio JM, Doncel Vecino LJ et al (2014) A prospective randomised study of the paclitaxel-coated balloon catheter in bifurcated coronary lesions (BABILON trial): 24-month clinical and angiographic results. EuroIntervention 10(1):50–57
Belkacemi A, Agostoni P, Nathoe HM et al (2012) First results of the DEB-AMI (drug eluting balloon in acute ST-segment elevation myocardial infarction) trial: a multicenter randomized comparison of drug-eluting balloon plus bare-metal stent versus bare-metal stent versus drug-eluting stent in primary percutaneous coronary intervention with 6-month angiographic, intravascular, functional, and clinical outcomes. J Am Coll Cardiol 59(25):2327–2337
Kleber FX, Rittger H, Bonaventura K et al (2013) Drug-coated balloons for treatment of coronary artery disease: updated recommendations from a consensus group. Clin Res Cardiol 102(11):785–797
Sim HW, Ananthakrishna R, Chan SP et al (2018) Treatment of very small de novo coronary artery disease with 2.0 mm drug-coated balloons showed 1-year clinical outcome comparable with 2.0 mm drug-eluting stents. J Invasive Cardiol 30(7):256–261
Sinaga DA, Ho HH, Watson TJ et al (2016) Drug-coated balloons: a safe and effective alternative to drug-eluting stents in small vessel coronary artery disease. J Interv Cardiol 29(5):454–460
Cortese B, Silva Orrego P, Agostoni P et al (2015) Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv 8(15):2003–2009
Hayıroğlu M, Keskin M, Uzun AO et al. Predictors of in-hospital mortality in patients with ST-segment elevation myocardial Infarction complicated with cardiogenic shock. Heart Lung Circ. 2017
Unverdorben M, Kleber FX, Heuer H et al (2010) Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol 99(3):165–174
Vranckx P, Cutlip DE, Mehran R et al (2010) Myocardial infarction adjudication in contemporary all-comer stent trials: balancing sensitivity and specificity. Addendum to the historical MI definitions used in stent studies. EuroIntervention 5(7):871–874
Zeymer U, Waliszewski M, Spiecker M et al (2014) Prospective ‘real world’ registry for the use of the ‘PCB only’ strategy in small vessel de novo lesions. Heart 100(4):311–316
Venetsanos D, Lawesson SS, Panayi G et al. Long-term efficacy of drug coated balloons compared with new generation drug-eluting stents for the treatment of de novo coronary artery lesions. Catheter Cardiovasc Interv. 2018
Mahmood Zuhdi AS, Zeymer U, Waliszewski M et al (2016) The use of paclitaxel coated balloon (PCB) in acute coronary syndrome of small vessel de novo lesions: an analysis of a prospective ‘real world’ registry. Springerplus 5:373
Poerner TC, Duderstadt C, Goebel B, Kretzschmar D, Figulla HR, Otto S (2017) Fractional flow reserve-guided coronary angioplasty using paclitaxel-coated balloons without stent implantation: feasibility, safety and 6-month results by angiography and optical coherence tomography. Clin Res Cardiol 106(1):18–27
Widder JD, Cortese B, Levesque S et al. Coronary artery treatment with an urea-based paclitaxel-coated balloon: the European wide Falcon all comers DCB Registry (FALCON-Registry). EuroIntervention. 2018
Cortese B, Berti S, Biondi-Zoccai G et al (2014) Drug-coated balloon treatment of coronary artery disease: a position paper of the Italian Society of Interventional Cardiology. Catheter Cardiovasc Interv 83(3):427–435
Kleber FX, Schulz A, Waliszewski M et al (2015) Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol 104(3):217–225
Acknowledgements
We would like to thank all professors and study nurses involved in the DEBATE study.
Funding
This work was financially supported by Program for National Science Funds of China (Grant No. 81730011), National key R & D plan (Grant No. 2018YFA0107400), Program for Changjiang Scholars and Innovative Research Teamin University (Grant No. PCSIRT-14R08).
Author information
Authors and Affiliations
Contributions
All of the authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Ethical approval
This study was approved by the local ethics committee of our institution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Zhang, YJ., Deng, LX. et al. 12-Month clinical results of drug-coated balloons for de novo coronary lesion in vessels exceeding 3.0 mm. Int J Cardiovasc Imaging 35, 579–586 (2019). https://doi.org/10.1007/s10554-018-1505-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-018-1505-z