Abstract
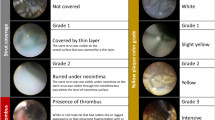
Polymeric component is associated with the increased risk of delayed vessel healing and stent endothelialization. We aimed to clarify neointimal coverage within 1 month after implantation of the new-generation abluminal biodegradable polymer (BP) drug-eluting stent (DES) compared with the second-generation durable polymer (DP) everolimus-eluting stent (EES). Between November 2015 and October 2016, 32 BP-DES and 25 DP-EES were evaluated by optical frequency domain imaging (OFDI) within 1 month after the procedure. The average interval to follow-up OFDI was not significantly different between the groups (16.3 ± 7.7 days in BP-DES vs. 15.4 ± 7.4 days in DP-EES, P = 0.75). Neointimal coverage was significantly superior in BP-DES in both apposed and malapposed strut (apposed: 53.9% in BP-DES vs. 28.0% in DP-EES, P < 0.001; malapposed: 22.9% in BP-DES vs. 7.5% in DP-EES, P = 0.001). When the follow-up period was divided into < 2 and > 2 weeks, neointimal coverage was also significantly superior in BP-DES (< 2 weeks: 47.7% in BP-DES vs. 19.2% in DP-EES, P < 0.001; > 2 weeks: 60.1% in BP-DES vs. 37.4% in DP-EES, P = 0.001). The new-generation BP-DES showed excellent early neointimal coverage compared with the second-generation DP-EES in both apposed and malapposed struts.





Similar content being viewed by others
References
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E et al (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48:193–202
Finn AV, Joner M, Nakazawa G, Kolodgie F, Newell J, John MC et al (2007) Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation 115:2435–2441
Raber L, Magro M, Stefanini GG, Kalesan B, van Domburg RT, Onuma Y et al (2012) Very late coronary stent thrombosis of a newer-generation everolimus-eluting stent compared with early-generation drug-eluting stents: a prospective cohort study. Circulation 125:1110–1121
Stefanini GG, Byrne RA, Serruys PW, de Waha A, Meier B, Massberg S et al (2012) Biodegradable polymer drug-eluting stents reduce the risk of stent thrombosis at 4 years in patients undergoing percutaneous coronary intervention: a pooled analysis of individual patient data from the ISAR-TEST 3, ISAR-TEST 4, and LEADERS randomized trials. Eur Heart J 33:1214–1222
Saito S, Valdes-Chavarri M, Richardt G, Moreno R, Iniguez Romo A, Barbato E et al (2014) A randomized, prospective, intercontinental evaluation of a bioresorbable polymer sirolimus-eluting coronary stent system: the CENTURY II (clinical evaluation of new terumo drug-eluting coronary stent system in the treatment of patients with coronary artery disease) trial. Eur Heart J 35:2021–2031
Kereiakes DJ, Meredith IT, Windecker S, Lee Jobe R, Mehta SR, Sarembock IJ et al (2015) Efficacy and safety of a novel bioabsorbable polymer-coated, everolimus-eluting coronary stent: the EVOLVE II randomized trial. Circ Cardiovasc Interv 8:e002372
de la Torre Hernandez JM, Tejedor P, Camarero TG, Duran JM, Lee DH, Monedero J et al (2016) Early healing assessment with optical coherence tomography of everolimus-eluting stents with bioabsorbable polymer (synergy) at 3 and 6 months after implantation. Catheter Cardiovasc Interv 88:E67–E73
Miyoshi N, Shite J, Shinke T, Otake H, Tanino Y, Ogasawara D et al (2010) Comparison by optical coherence tomography of paclitaxel-eluting stents with sirolimus-eluting stents implanted in one coronary artery in one procedure: 6-month follow-up. Circ J 74:903–908
Takano M, Murakami D, Yamamoto M, Kurihara O, Murai K, Inami T et al (2013) Six-month follow-up evaluation for everolimus-eluting stents by intracoronary optical coherence tomography: comparison with paclitaxel-eluting stents. Int J Cardiol 166:181–186
Takano M, Inami S, Jang IK, Yamamoto M, Murakami D, Seimiya K et al (2007) Evaluation by optical coherence tomography of neointimal coverage of sirolimus-eluting stent three months after implantation. Am J Cardiol 99:1033–1038
Eppihimer MJ, Sushkova N, Grimsby JL, Efimova N, Kai W, Larson S et al (2013) Impact of stent surface on thrombogenicity and vascular healing: a comparative analysis of metallic and polymeric surfaces. Circ Cardiovasc Interv 6:370–377
Barlis P, Regar E, Serruys PW, Dimopoulos K, van der Giessen WJ, van Geuns RJ et al (2010) An optical coherence tomography study of a biodegradable vs. durable polymer-coated limus-eluting stent: a LEADERS trial sub-study. Eur Heart J 31:165–176
Tada T, Kastrati A, Byrne RA, Schuster T, Cuni R, King LA et al (2014) Randomized comparison of biolimus-eluting stents with biodegradable polymer versus everolimus-eluting stents with permanent polymer coatings assessed by optical coherence tomography. Int J Cardiovasc Imaging 30:495–504
Kubo T, Akasaka T, Kozuma K, Kimura K, Fusazaki T, Okura H et al (2014) Vascular response to drug-eluting stent with biodegradable vs. durable polymer: optical coherence tomography substudy of the NEXT. Circ J 78:2408–2414
Kitabata H, Kubo T, Komukai K, Ishibashi K, Tanimoto T, Ino Y et al (2012) Effect of strut thickness on neointimal atherosclerotic change over an extended follow-up period (>/= 4 years) after bare-metal stent implantation: intracoronary optical coherence tomography examination. Am Heart J 163:608–616
Kuramitsu S, Kazuno Y, Sonoda S, Domei T, Jinnouchi H, Yamaji K et al (2016) Vascular response to bioresorbable polymer sirolimus-eluting stent vs. permanent polymer everolimus-eluting stent at 9-month follow-up: an optical coherence tomography sub-study from the CENTURY II trial. Eur Heart J Cardiovasc Imaging 17:34–40
Lee R, Foin N, Ng J, Allen J, Soh N, Ang I et al (2016) Early coverage of drug-eluting stents analysed by optical coherence tomography: evidence of the impact of stent apposition and strut characteristics on the neointimal healing process. EuroIntervention 12:e605–e614
Gutierrez-Chico JL, Alegria-Barrero E, Teijeiro-Mestre R, Chan PH, Tsujioka H, de Silva R et al (2012) Optical coherence tomography: from research to practice. Eur Heart J Cardiovasc Imaging 13:370–384
Murata A, Wallace-Bradley D, Tellez A, Alviar C, Aboodi M, Sheehy A et al (2010) Accuracy of optical coherence tomography in the evaluation of neointimal coverage after stent implantation. JACC Cardiovasc Imaging 3:76–84
Acknowledgements
The authors are indebted to Dr. Edward Barroga (http://orcid.org/0000-0002-8920-2607) for editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no financial relationships or other conflicts of interest relevant to the contents of this paper.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kobayashi, N., Ito, Y., Yamawaki, M. et al. Very early neointimal coverage of new biodegradable polymer drug-eluting stent compared with durable polymer everolimus-eluting stent evaluated by optical frequency domain imaging. Int J Cardiovasc Imaging 34, 515–522 (2018). https://doi.org/10.1007/s10554-017-1273-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-017-1273-1