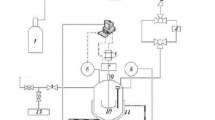
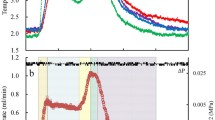
The study of the decomposition process of gas hydrates at atmospheric pressure and temperatures below 0°C revealed that methanol could affect this process in different ways, depending on its saturation with environmental components. Indeed, due to the absorption of methane from the hydrate by methanol, the onset of its decomposition is observed at lower temperatures. Nevertheless, decomposition proceeds more slowly than with pure methane hydrate. When the methanol surrounding the methane hydrate is saturated with other medium components, the hydrate dissociation occurs at the equilibrium temperature (when intersecting the hydrate–ice–gas curve in a system without additives) regardless of the alcohol concentration. A similar situation is observed with hydrate obtained from a methane-propane gas mixture; however, under experimental conditions, ice begins to melt at a lower temperature compared to the dissociation point of methane-propane hydrate (in the case of methane hydrate, the situation is reversed: the hydrate is less stable). High concentrations of methanol (above 40 mass%) lead to a significant decrease in the temperature of hydrate decomposition. The data obtained show that methanol in low dosages (about 10 mass%) can be used for gas storage and transportation since, under certain conditions, it does not shift the equilibrium curve of hydrate formation and slows down the process of methane hydrate decomposition.


Similar content being viewed by others
References
R. J. Davey, Polymorphism in Molecular Crystals Joel Bernstein, Oxford University Press, New York (2002). Joel Bernstein, Book: Polymorphism in Molecular Crystals, Oxford University Press, New York (2002).
Y. Wang, S. Subramanian, D. Estanga, et al., Energy & Fuels, 34, No. 11, 13523-13535 (2020).
A. S. Stoporev, A. A. Sizikov, T. V. Cheshkova, et al., Energy & Fuels, 32, No. 11, 11279-11288 (2018).
M. A. Sayed, R. K. Saini, E. AlAli, et al., Energy & Fuels, 35, No. 17, 13731-13742 (2021).
A. Y. Manakov, A. S. Stoporev, Russian Chemical Reviews, 90, No. 5, 566 (2021).
Z. R. Chong, S. H. B. Yang, P. Babu, et al., Applied Energy, 162, 1633-1652 (2016).
E. Chuvilin, V. Ekimova, B. Bukhanov, et al., Geosciences, 9, No. 4, 188 (2019).
E. Chuvilin, V. Ekimova, D. Davletshina, et al., Energy & Fuels, 36, No. 18, 10519-10528 (2022).
M. Lauricella, M. R. Ghaani, P. K. Nandi, et al., The Journal of Physical Chemistry C, 126, No. 13, 6075-6081 (2022).
T. V. Rodionova, A. A. Sizikov, V. Y. Komarov, et al., The Journal of Physical Chemistry B, 121, No. 18, 4900-4908 (2017).
E. D. Sloan, C. A. Koh, Clathrate Hydrates of Natural Gases, Third edition, Boca Rator, CRC Press, London–New York (2008).
M. S. Wahl, A. Aasen, D. R. Hjelme, et al., Fluid Phase Equilibria, 522, 112741.
H. Sharifi, A. Yoneyama, S. Takeya, et al., The Journal of Physical Chemistry C, 122, No. 30, 17019–17023 (2018).
S. Takeya, S. Muromachi, A. Yoneyama, et al., The Journal of Physical Chemistry Letters, 13, No. 9, 2130-2136 (2022).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 6, pp. 40–43 November – December, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yarakhmedov, M.B., Kiyamov, A.G., Semenov, M.E. et al. Features of the Decomposition of Gas Hydrates in the Presence of Methanol at Atmospheric Pressure. Chem Technol Fuels Oils 58, 957–961 (2023). https://doi.org/10.1007/s10553-023-01475-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-023-01475-y