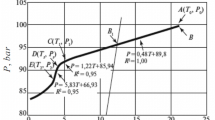
A study was carried out on combined inhibition by a solution containing 0.5% polymer kinetic inhibitor (KI) + 10.0% methanol as a thermodynamic inhibitor (TI) in the formation of methane hydrate (Class I) and the hydrate of 95.66 mol. % CH4 + 4.34 mol.% C3H8 (methanepropane mixture) (Class II). This combined inhibition was studied by an isothermal method and a method entailing cooling at a constant rate using a GHA350 autoclave. Methane was shown to have an adverse effect on the inhibiting properties of the polymer KI both relative to formation of methane hydrate and hydrates of C1 –C3 hydrocarbons. The loss of inhibition by the polymer KI in the presence of methanol is expressed as the decrease in the extent of supercooling attainable in the system without adsorption of the hydrate-forming gas (1.5–2.5°C in comparison with the system without TI). The induction time is shown to depend on the extent of supercooling in the system during inhibition of formation of the Class I and Class II hydrates by the solution containing 0.5% KI + 10.0% TI.




Similar content being viewed by others
References
V. A. Istomin and V. G. Kvon, Prevention and Elimination of Gas Hydrates in Gas Production Systems [in Russian], IRTs Gazprom, Moscow (2004).
H. J. Ng, C. J. Chen, and T. Saeterstad, Fluid Phase Equilibria, 36, 99–106 (1987).
K. Y. Song and R. Kobayashi, Fluid Phase Equilibria, 47(2), 295–308 (1989).
H. Haghighi, et al., Fluid Phase Equilibria, 276(1), 24–30 (2009).
J. P. Lederhos, et al., Chemical Engineering Science, 51(8), 1221–1229 (1996).
E. D. Sloan and C. A. Koh, Clathrate Hydrates of Natural Gases, 3rd edition, CRC Press/Francis & Taylor, Boca Raton, FL (2008).
M. A. Kelland, Energy & Fuels, 20, 825-847 (2006).
A. Perrin, O. M. Musa, and J. W. Steed, Chemical Society Reviews, 42(5), 1996–2015 (2013).
B. Kvamme, T. Kuznetsova, and K. Aasoldsen, Journal of Molecular Graphics and Modelling, 23(6), 524–536 (2005).
B. J. Anderson, et al, Journal of the American Chemical Society, 127(50), 17852–17862 (2005).
A. P. Semenov, V. I. Medvedev, P. A. Gushchin, et al., Khimiya i Tekhnologiya Topliv i Masel, 51, No. 6, 2429 (2015).
J. A. Moore, in: Offshore Technology Conference, Houston, TX, May 47, 2009, OTC 19869.
C. Xiao and H. Adidharma, Chemical Engineering Science, 64(7), 1522–1527 (2009).
C. Xiao, N. Wibisono, and H. Adidharma, Chemical Engineering Science, 65(10), 3080–3087 (2010).
A. R. Richard and H. Adidharma, Chemical Engineering Science, 87, 270–276 (2013).
K. S. Kim, J. W. Kang, and S. P. Kang, Chemical Communications, 47(22), 6341–6343 (2011).
S. Park, et al., Energy & Fuels, 27(11), 6581–6586 (2013).
E. D. Sloan, et al., Industrial & Engineering Chemistry Research, 37(8), 3124–3132 (1998).
M. Cha, et al., Chemical Engineering Science, 99, 184–190 (2013).
B. Tohidi, et al., Energy & Fuels, 29(12), 8254–8260 (2013).
J. Kim, The Journal of Physical Chemistry B, 118(30), 9065–9075 (2014).
A. P. Semenov, V. I. Medvedev, P. A. Gushchin, et al., Khimiya i Tekhnologiya Topliv i Masel, 52, No. 1, 2933 (2016).
H. Ajiro, et al., Energy & Fuels, 24(12), 6400–6410 (2010).
P. C. Chua, et al., Energy & Fuels, 26(8), 4961–4967 (2012).
P. C. Chua, et al., Energy & Fuels, 27(1), 183–188 (2012).
J. M. Cohen, P. F. Wolf, and W. D. Young, Energy & Fuels, 12(2), 216–218 (1998).
T. M. Svartaas, M. A. Kelland, and L. Dybvik, Annals of the New York Academy of Sciences, 912(1), 744–752 (2000).
V. I. Medvedev, et al., Chemistry and Technology of Fuels and Oils, 51(5), 470–479 (2015).
E. D. Sloan, The Hydrate Prediction Program Csmhyd 2.0 (1998).
P. Gayet, C. Dicharry, G. Marion, et al., ChemicalEngineeringScience, 60, 5751–5758 (2005).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 2, pp. 29 – 33, March – April, 2016.
Rights and permissions
About this article
Cite this article
Semenov, A.P., Medvedev, V.I., Gushchin, P.A. et al. Polymer–Methanol Combines Inhibition of Gas Hydrate Formation. Chem Technol Fuels Oils 52, 162–170 (2016). https://doi.org/10.1007/s10553-016-0686-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-016-0686-1