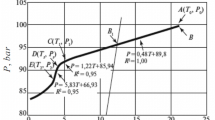
We have studied the inhibiting properties of the reagents Luvicap 55W and Luvicap EG in the process of KC-I methane hydrate formation in the pressure range 60-120 bar. In order to evaluate the effectiveness of the kinetic inhibition, we used the method of cooling at a constant rate of 1 degree/hour. We established that both in a system containing the kinetic inhibitor and in a system without the inhibitor, the initial pressure does not affect the maximum degree of supercooling at which methane hydrate is still not formed. The reagents Luvicap 55W and Luvicap EG at a concentration of 0.5 wt.% can effectively inhibit methane hydrate formation for a degree of supercooling no greater than 6°C-7°C. For inhibition of KC-I hydrate formation for a greater degree of supercooling, the reagents Luvicap 55W and Luvicap EG should be used in a higher concentration or combined with appropriate thermodynamic inhibitors.

Similar content being viewed by others
References
M. A. Kelland, Energy & Fuels, 20, 825-847 (2006).
E. D. Sloan and C. A. Koh, Clathrate Hydrates of Natural Gases (3rd ed.). CRC Press/Taylor & Francis, Boca Raton, FL (2008).
M. A. Kelland, in: Advances in Materials Science Research (Ed: M. C. Wytherst), Nova Science Publishers Inc., New York (2011), Vol. 8, Chapter 5.
M. A. Kelland, F. T. Reyes, and K. V. Trovik, Chem. Eng. Sci., 93, 423-428 (2013).
C. Nakarit, M. A. Kelland, D. Liu, et al, Ibid., 102, 424-431 (2013).
F. T. Reyes, E. L. Malines, C. R. Beker, et al., Energy & Fuels, 27, 3154-3160 (2013).
F. T. Reyes, L. Guo, G. V. Hedgepeth, et al., in: Proc. 8th Internat. Conf. on Gas Hydrates (ICGH8–2014), Beijing, China, July 28 – August 1, 2014.
C. D. Magnusson and M. A. Kelland, Ibid.
S. Al-Adel, J. A. G. Dick, R. El-Ghafari, et al., Fluid Phase Equilibria, 267, 92-98 (2008).
P. Gayet, C. Dicharry, G. Marion, et al., Chem. Eng. Sci., 60, 5751-5758 (2005).
This research was performed with financial support from the Ministry of Science and Education of the Russian Federation (Grant No. 14.574.21.0052, Identification No. RFMEFI57414X0052).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 6, pp. 30 – 32, November – December, 2014.
Rights and permissions
About this article
Cite this article
Semenov, A.P., Medvedev, V.I., Mikhailov, S.B. et al. Effect of Pressure on the Effectiveness of the Kinetic Inhibition of Hydrate Formation by Polymer Reagents. Chem Technol Fuels Oils 50, 489–493 (2015). https://doi.org/10.1007/s10553-015-0554-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-015-0554-4