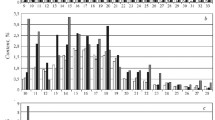
We have studied the effect of catalytic aquathermolysis of heavy oil in the presence of a hydrogen donor (formic acid) on the viscosity of Liaohe extra-heavy oil. The paraffinic/naphthenic and aromatic hydrocarbon content of the oil as well as the H:C ratio of the oil increase after aquathermolysis in the presence of a hydrogen donor, while the sulfur, resin, and asphaltene content decreases dramatically. We use the thermogravimetric method to show that with aquathermolysis in the presence of formic acid, a substantial portion of the asphaltenes in the heavy oil is converted to paraffins. Accelerating the aquathermolysis reaction results in a synergistic effect between the catalyst and the hydrogen donor.




Similar content being viewed by others
References
Z. X. Fan, F. L. Zhao, J. X. Wang, and Y. G. Gong, “Upgrading and viscosity reduction of extra heavy oil by aquathermolysis with hydrogen donor,” Journal of Fuel Chemistry and Technology, 34, No. 3, 315-318 (2006).
F. J. Zhao, Y. J. Liu, T. H. Zhao, S. B. Wen, and G. Zhao, “Advance in catalytically upgrading heavy oil by aquathermolysis using hydrogen donor,” Oilfield Chemistry, 2, No. 4, 379-384 (2006).
F. J. Zhao, Y. J. Liu, G. Zhao, and S. B. Hu, “Hydrogen donor catalytic upgrading for heavy oil and asphaltene pyrolysis under the conditions of steam injection,” Chemical Industry and Engineering Progress, 27, No. 9, 1453-1459 (2008).
J. C. Bricker, C. C. Nagel, C. C. Bhattacharyya, and S. G. Shore, “Hydride donating properties of [HRu3(CO)11] -in the presence of carbon monoxide, chemistry of ruthenium carbonyl anions relevant to the catalysis of the water gas shift reaction,” Journal of the American Chemical Society, 107, No. 2, 377-384 (1985).
R. H. Fish, and J. J. Komlenic, “Molecular characterization and profile identifications of vanadyl compounds in heavy crude petroleums by liquid chromatography/graphite furnace atomic spectrometry,” Analytical Chemistry, 56, 511 (1984).
Y. L. Chen, Y. Q. Wang, C. Wu, and F. Xia, “Laboratory experiments and field tests of an amphiphilic metallic chelate for catalytic aquathermolysis of heavy oil,” Energy & Fuels, 22, No. 3, 1501-1508 (2008).
Y. L. Chen, Y. Q. Wang, J. Y. Lu, and C. Wu, “The viscosity reduction of nano-keggin-K3PMo12O40 in catalytic aquathermolysis of heavy oil,” Fuel, 88, No. 8, 1426-1434 (2009).
H. F. Fan, Y. J. Liu, X. F. Zhao, and L. G. Zhong, “Studies on effect of metal ions on aquathermolysis reaction of Liaohe heavy oils under steam treatment [J],” Journal of Fuel Chemistry and Technology, 29, 430-433 (2001).
C. Ovalles, C. Vallejos, T. Vasquez, J. Martinis, A. Perez-Perez, E. Cotte, L. Castellanos, and H. Rodriguez, “Extra-heavy crude oil downhole upgrading process using hydrogen donor under steam injection conditions,” in: SPE International Thermal Operations and Heavy Oil Symposium, Margarita Island, March (2001), SPE69692.
G. S. Hewgill and L. G. Kalfayan, “Enhanced oil recovery technique using hydrogen precursors,” US Patent 5,105,887 (1992).
C. Ovalles, C. Vallejos, T. Vasquez, I. Rojas, U. Ehrman, J. L. Benitez, and R. Martinez, “Downhole upgrading of extra-heavy crude oil using hydrogen donors and methane under steam injection conditions,” Petroleum Science and Technology, 21, Nos. 1 & 2, 255-274 (2003).
P. D. Clark, J. B. Hyne, and J. D. Tyrer, “Chemistry of organosulfur compound type occurring in heavy oil sands. 2. Influence of pH on the high temperature hydrolysis of tetrahydrothiophene and thiophene,” Fuel, 63, No. 1, 125-128 (1984).
E. J. Hoffman, “Hydrogen for the enhanced recovery of heavy crudes,” Energy Sources, 11, No. 4, 263-272 (1989).
E. Y. Chen, Y. J. Liu, and S. B. Wen, “Heavy oil viscosity reduction by using toluene as additive for catalytic aquathermolysis reaction,” Journal of Daqing Petroleum Institute, 6, No. 29, 38-39 (2005).
P. D. Clark and J. B. Hyne, “Studies on the chemical reactions of heavy oils under steam stimulation condition,” AOSTRA J. Res., 29, No. 6, 29-39 (1990).
J. B. Hyne, Synopsis Report No. 50, “Aquathermolysis,” AOSTRA Contracts No. 11103103B/C (1986).
J. B. Hyne and J. D. Tyrer, “Use of hydrogen-free carbon monoxide with steam in recovery of heavy oil at low temperatures,” US Patent 4487264 (1984).
This work was done with the support of the National Natural Science Foundation of China (Project No. 2146002) and within the Major National Special Projects for Oil and Gas (Project No. 2008ZX05012-001).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 4, pp. 16 – 21, July – August, 2012.
Rights and permissions
About this article
Cite this article
Zhao, F., Liu, Y., Wu, Y. et al. Study of catalytic aquathermolysis of heavy oil in the presence of a hydrogen donor. Chem Technol Fuels Oils 48, 273–282 (2012). https://doi.org/10.1007/s10553-012-0368-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-012-0368-6