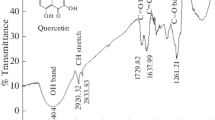
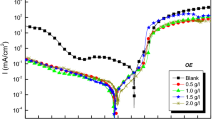
We used electrochemical techniques (galvanostatic and potentiodynamic anodic polarization methods and also electrochemical impedance spectroscopy) to study corrosion inhibition by some natural oils (parsley, lettuce, sesame, arugula, and sweet almond oils) on the corrosion of type 304 stainless steel in 0.1 M NaOH solution. We show that the inhibition efficiency increases as the concentration of these oils increases. The inhibiting effect was attributed to adsorption of the major components of these oils on the stainless steel surface. The adsorption process is described by a Langmuir isotherm. It was found that incorporation of chloride ions in a 0.1 M NaOH solution accelerates pitting corrosion of the stainless steel as a result of shifting the pitting potential toward more negative values. Addition of the natural oils under study to sodium hydroxide solutions containing chloride ions shifts the pitting potential toward more positive values, indicating increased resistance of the steel to pitting corrosion. As the concentration of the oils under study in the solution increases, the charge transfer resistance increases while the capacitance of the double layer decreases.









Similar content being viewed by others
References
Xian Ghong Li, Schuduan Dang, and Hui Fu, “Three pyrazine derivatives as corrosion inhibitors for steel in 1.0 M H2SO4 solution,” Corros. Sci., 53, 3241 (2011).
M. Abdallah, “ Behavior of 304 stainless steel in sulphuric acid solution and its inhibition by some substituted pyrazolones,” Mater. Chem. and Phys., 82, 768 (2003).
A. S. Fouda, M. Abdallah, S. M. Al-Ashrey, and A. A. Abdel-Fattah, “Some crown ethers as inhibitors for corrosion of stainless steel 304 in aqueous solution,” Desalination, 250, 538 (2010).
A. S. Fouda, “Inhibition effect of phenylthiazole derivatives on corrosion of 304 L stainless steel in HCl solution,” Corros. Sci., 51, 868 (2009).
X. L. Cheng, H. Y. Ma, S. H. Chen, R. Yu, X. Chen, and Z. M. Yao, “Corrosion of stainless steels in acid solutions with sulfur-containing compounds,” Corros. Sci., 41, 321 (1999).
S. A. Abd El-Maksoud and A. S. Fouda, “Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium,” Mater. Chem and Phys., 93, 84 (2005).
A. Singh, I. Ahamad, V. K. Singh, and M. A. Quraishi, “Inhibition effect of environmentally benign karanj (Pongamia Pinnata) seed extract on corrosion of mild steel in hydrochloric acid solution,”, J. Solid State Electrochem., 15, 1087 (2011).
M. Abdallah, S. O. Al-Karanee, and A. A. Abdel-Fattah, “Inhibition of acidic and pitting corrosion of nickel using natural black cumin oil,” Chem. Eng. Comm., 197, 1446 (2010).
M. Abdallah, S. O. Al-Karanee, and A. A. Abdel-Fattah, “Inhibition of corrosion of nickel and its alloys by natural clove oil,” Chem. Eng. Comm., 196, 1406 (2009).
M. Abdallah, M. A. Radwan, S. M. Shohayeb, and S. Abdelhamed, “Use of some natural oils as crude pipeline corrosion inhibitors in sodium hydroxide solutions,” Chemistry and Technology of Fuels and Oils, 46, No. 5, 354 (2010).
A. Y. El-Etre, “Inhibition of acid corrosion of carbon steel using aqueous extract of olive leaves,” J. Colloid Interface Sci., 314, 578 (2007).
T. Tsuru, S. Haruyama, and G. Boshoku, “Basics of corrosion measurements,” J. Japan Soc. Corros. Eng., 27, 573 (1978).
E. B. Truitt, G. Duritz, and E. M. Ebersberger, “Evidence of monoamine oxidase inhibition by myristicin and nutmeg,” Proc. Soc. Exp. Biol. Med., 112, 647 (1963).
Richard D. Hubant, J. Agric. Food Chem., 54, No. 7, 2678 (2006).
M. Kliskic, J. Radosevic, and S. Gndic, J. Appl. Electrochem., 27, 947 (1994).
M. Abdallah, “Tetradecyl-1,2-diol propenoxylates as inhibitors for corrosion of aluminum in hydrochloric acid,” Bull. Electrochem., 16, No. 6, 258 (2000).
T. P. Hoar and G. G. Wood, J. Electrochim. Acta, 7, 333 (1964).
M. Metikos-Hukovic, R. Bobic, Z. Gwabac, and S. Brinic, J. Appl. Electrochem., 24, 772 (1994).
A. Caprani, I. Epelboin, Ph. Morel, and H. Takenouti, in: Proceedings of the Fourth European Symposium On Corrosion Inhibitors, Ferrara, Italy (1975), p. 571.
I. Epelboin, M. Keddam, and H. Takenouti, J. Appl. Electrochem., 2, 71 (1972).
I. Sekine, M. Sabongi, H. Hagiuda, T. Oshibe, M. Yuasa, T. Imahc, Y. Shibata, and T. Wake, J. Electrochem. Soc., 139, 3167 (1992).
X. Li, S. Deng, H. Fen, and T. Li, Electrochim. Acta, 54, 4089 (2009).
A. S. Fouda, M. Mousa, F. Taha, and A. I. Eleanaa, J. Corros. Sci., 26, 719 (1986).
M. Abdallah, “Antibacterial drugs as corrosion inhibitors of corrosion of aluminum in hydrochloric acid solution,” Corros. Sci., 46, 1981 (2004).
F. M. Al-Nowaiser, M. Abdallah, and E. H. El-Mossalamy. E. H., “Rosemary oil as a corrosion inhibitor of carbon steel in 0.5 M sulfuric acid solution,” Chemistry and Technology of Fuels and Oil, 47, No. 1, 66 (2011).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 3, pp. 46 – 52, May – June, 2012.
Rights and permissions
About this article
Cite this article
Abdallah, M., Zaafarany, I., Khairou, K.S. et al. Natural oils as corrosion inhibitors for stainless steel in sodium hydroxide solutions. Chem Technol Fuels Oils 48, 234–245 (2012). https://doi.org/10.1007/s10553-012-0364-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-012-0364-x