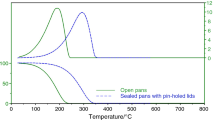
Use of pour depressants is the most effective and economical method of improving the low-temperature properties of diesel fuels. The reaction of T1804D pour depressant with different solvents was investigated by differential scanning calorimetry. It was shown that they affect the decrease in the limiting filterability temperature and the solid point of diesel fuel to a different degree. The effectiveness of the pour depressant increased when it was mixed with aromatic solvents that ensure dissolution and dispersion of wax crystals in diesel fuel at low temperatures.


Similar content being viewed by others
References
US Patent No. 6593426.
US Patent No. 6017370.
Zhang Jin-li, Wu Chuanjie, Li Wei, et al., Fuel, 82, 1419-1426 (2003).
Chuanjie Wuk, Jin-li Zhang, Wei Li, et al., Fuel, 84, 2039-2047 (2005).
R. A. Soldi, A. R. S. Oliveira, R. V. Barbosa, et al., Eur. Polym. J., 43, 3671-3678 (2007).
Cuiyu Jiang, Ming Xu, and Xiaoli Xi, J. Nat. Gas Chem., 15, 217-222 (2006).
Sheng Han, Yuping Song, and Tianhui Ren, Energy Fuels, 23, 2576-2580 (2009).
Liu Shufeng and Wang Shujun, Adv. Fine Petrochem., 2, No. 4, 33-36 (2001).
Huang Ying-xiong, Shandong Chem. Ind., 37, 20-22 (2008).
Zhao Guang-hui, Guan Xu, Cui Xi-hong, et al., Chem. Intermed., 10, 15-19 (2007).
N. M. Ribeiro, A. C. Pinto, C. M. Quintella, et al., Energy Fuels, 21, 2433-2445 (2007).
L. V. Castro and F. Fazquez, Ibid., 22, 4006-4011 (2008).
C. D. Gamlin, N. K. Dutta, N. R. Choudhury, et al., Thermochim. Acta, 392-393, 357-369 (2002).
A. A. Hafiz and T. T. Khidr, J. Petrol. Sci. Eng., 56, 296-302 (2007).
L. C. Machado, E. F. Lucas, and G. Gonzalez, Ibid., 32, 159-165 (2001).
I. M. El-Gamal, Physicochem. Eng. Aspects, 135, 283-291 (1998).
A. Jukic, M. Rogosic, and Z. Janovic, Eur. Polym. J., 42, 1105-1112 (2006).
W. H. Chen, X. D. Zhang, Z. C. Zhao, et al., Fluid Phase Equilibr., 280, 9-15 (2009).
Cai Zhi, Huang Wei-qiu, and Li Wei-ming, Beijing China Petrochem. Press, 20-23 (2005).
Zhang Hong-xi, Xie Chen-xi, and Chen Zhao-hui, J. Xinjiang Univ. (Natural Science Ed.), 21, No. 3, 282284 (2004).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya i Tekhnologiya Topliv i Masel, No. 6, pp. 11 – 15, 2010.
Rights and permissions
About this article
Cite this article
Han, S., Zeng, K., Shen, S. et al. Reaction of pore depressants and solvents. Chem Technol Fuels Oils 46, 378–384 (2011). https://doi.org/10.1007/s10553-011-0238-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10553-011-0238-7