Abstract
Objective
Nocturnal rodents are frequently used as models in human breast cancer research, but these species have very different visual and circadian systems and, therefore, very different responses to optical radiation or, informally, light. Because of the impact of light on the circadian system and because recent evidence suggests that cancer risk might be related to circadian disruption, it is becoming increasingly clear that optical radiation must be properly characterized for both nocturnal rodents and diurnal humans to make significant progress in unraveling links between circadian disruption and breast cancer. In this paper, we propose a quantitative framework for comparing radiometric and photometric quantities in human and rodent studies.
Methods
We reviewed published research on light as a circadian stimulus for humans and rodents. Both suppression of nocturnal melatonin and phase shifting were examined as outcome measures for the circadian system.
Results
The data were used to develop quantitative comparisons regarding the absolute and spectral sensitivity for the circadian systems of humans and nocturnal rodents.
Conclusions
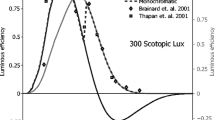
Two models of circadian phototransduction, for mouse and humans, have been published providing spectral sensitivities for these two species. Despite some methodological variations among the studies reviewed, the circadian systems of nocturnal rodents are approximately 10,000 times more sensitive to optical radiation than that of humans. Circadian effectiveness of different sources for both humans and nocturnal rodents are offered together with a scale relating their absolute sensitivities. Instruments calibrated in terms of conventional photometric units (e.g., lux) will not accurately characterize the circadian stimulus for either humans or rodents.
Similar content being viewed by others
Notes
Light, like time and mass, is a fundamental quantity, but unlike all other fundamental quantities, is defined in terms of a specific visual response in humans [11]. As such it is technically incorrect to refer to light when referring to other organisms, or in relation to nonvisual (e.g., circadian) responses in humans. The term optical radiation is preferred to describe the portion of the electromagnetic spectrum spanning ultraviolet, visible and infrared radiation. However, given the wide use of the term light to describe optical radiation in the biological and medical research community, the two terms are used interchangeably, albeit technically incorrectly, throughout this paper.
References
Chu KC, Tarone RE, Kessler LG etal. (1996) Recent trends in U.S. breast cancer incidence, survival, and mortality rates. J Natl Cancer Inst 88:1571–1579
Ghafoor A, Jemal A, Ward E, Cokkinides V, Smith R, Thun M (2003) Trends in breast cancer by race and ethnicity. CA Cancer J Clin 53:342–355
Steel CM, Cohen BB, Porter DE (1992) Familial breast cancer. Semin Cancer Biol 3:141–150
Stevens RG, Davis S (1996) The melatonin hypothesis: electric power and breast cancer. Environ Health Perspect 104(Suppl. 1):135–140
Jongmans W, Hall J (1999) Cellular responses to radiation and risk of breast cancer. Eur J Cancer 35:540–548
Cianfrocca M, Goldstein LJ (2004) Prognostic and predictive factors in early-stage breast cancer. Oncologist 9:606–616
Wiseman RA (2004) Breast cancer: critical data analysis concludes that estrogens are not the cause, however lifestyle changes can alter risk rapidly. J Clin Epidemiol 57:766–772
Hrushesky WJ (1985) Circadian timing of cancer chemotherapy. Science 228:73–75
Lis CG, Grutsch JF, Wood P, You M, Rich I, Hrushesky WJ (2003) Circadian timing in cancer treatment: the biological foundation for an integrative approach. Integr Cancer Ther 2:105–111
Stevens RG (1987) Electric power use and breast cancer: a hypothesis. Am J Epidemiol 125:556–561
Lennie P, Pokorny J, Smith VC (1993) Luminance. J Opt Soc Am A 10:1283–1293
Stevens RG, Wilson BW, Anderson LE (eds) (1997) The Melatonin Hypothesis: Breast Cancer and Use of Electric Power. Columbus, OH, Battelle Press
Dauchy RT, Blask DE, Sauer LA, Brainard GC, Krause JA (1999) Dim light during darkness stimulates tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett 144:131–136
Blask DE, Dauchy RT, Sauer LA, Krause JA, Brainard GC (2003) Growth and fatty acid metabolism of human breast cancer (MCF-7) xenografts in nude rats: impact of constant light-induced nocturnal melatonin suppression. Breast Cancer Res Treat 79:313–320
Davis S, Mirick DK, Stevens RG (2001) Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 93:1557– 1562
Hansen J (2001) Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst 93:1513–1515
Schernhammer ES, Laden F, Speizer FE etal. (2001) Rotating night shifts and risk of breast cancer in women participating in the nurses’ health study. J Natl Cancer Inst 93:1563–1568
Swerdlow A (2003) Shift Work and Breast Cancer Risk: A Critical Review of the Epidemiological Evidence, Research Report 132 Institute of Cancer Research, Surrey, UK
Schernhammer ES, Hankinson SE (2005) Urinary melatonin levels and breast cancer risk. J Natl Cancer Inst 97:1084– 1087
Stevens RG, Rea MS (2001) Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Cause Control 12:279–287
Filipski E, King VM, Li X etal. (2002) Host circadian clock as a control point in tumor progression. J Natl Cancer Inst 94:690– 697
Filipski E, King VM, Li X etal. (2003) Disruption of circadian coordination accelerates malignant growth in mice. Pathol Biol 51:216–219
Fu L, Lee CC (2003) The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer 3:350–361
Stevens RG (2005) Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiol 16:254–258
Pauley SM (2004) Lighting for the human circadian clock: recent research indicates that lighting has become a public health issue. Med Hypotheses 63:588–596
Reiter RJ (1985) Action spectra, dose-response relationships, and temporal aspects of light’s effects on the pineal gland. Anal NY Acad Sci 453:215–230
Lewy AJ, Wehr TA, Goodwin TK, Newsome DA, Markey SP (1980) Light suppresses melatonin secretion in humans. Science 210:1267–1269
McIntyre IM, Norman TR, Burrows GD, Armstrong SM (1989) Human melatonin suppression by light is intensity dependent. J Pineal Res 6:149–156
McIntyre IM, Norman TR, Burrows GD, Armstrong SM (1989) Quantal melatonin suppression by exposure to low intensity light in man. Life Sci 45:327–332
Bullough JD, Figueiro MG, Possidente BP, Parsons RH, Rea MS (2005) Additivity in murine circadian phototransduction. Zool Sci 22:223–227
Figueiro MG, Bullough JD, Parsons RH, Rea MS (2004) Preliminary evidence for spectral opponency in the suppression of melatonin by light in humans. NeuroReport 15:313–316
Rea MS, Bullough JD, Figueiro MG, Bierman A (2005) A model of phototransduction by the human circadian system. Brain Res Rev 50:213–228
Brainard GC, Richardson BA, Petterborg LJ, Reiter RJ (1982) The effect of different light intensities on pineal melatonin content. Brain Res 233:75–81
Lynch HJ, Deng MH, Wurtman RJ (1984) Light intensities required to suppress nocturnal melatonin secretion in albino and pigmented rats. Life Sci 35:841–847
Webb SM, Champney TH, Lewinski AK, Reiter RJ (1985) Photoreceptor damage and eye pigmentation: influence on the sensitivity of rat pineal N-acetyltransferase activity and melatonin levels to light at night. Neuroendocrinol 40:205–209
Thiele G, Holtorf A, Steinlechner S, Reiter RJ (1983) The influence of different light irradiances on pineal N-acetyltransferase activity and melatonin levels in the cotton rat, Sigmodon hispidus. Life Sci 33:1543–1547
Reiter RJ, Hurlbut EC, Brainard GC, Steinlechner S, Richardson BA (1983) Influence of light irradiance on hydroxyindole-O-methyltransferase activity, serotonin-N-acetyltransferase activity, and radioimmunoassayable melatonin levels in the pineal gland of the diurnally active Richardson’s ground squirrel. Brain Res 288:151–157
Reiter RJ, Steinlechner S, Richardson BA, King TS (1983) Differential response of pineal melatonin levels to light at night in laboratory-raised and wild-captured 13-lined ground squirrels (Spermophilus tridecemlineatus). Life Sci 32: 2625–2629
Reiter RJ, Peters JF (1984) Non-suppressibility by room light of pineal N-acetyltransferase activity and melatonin levels in two diurnally active rodents, the Mexican ground squirrel (Spermophilus mexicanus) and the eastern chipmunk (Tamias striatus). Endocr Res 10:113–121
Reiter RJ, King TS, Richardson BA, Hurlbut EC (1982) Studies on pineal melatonin levels in a diurnal species, the eastern chipmunk (Tamias striatus): effects of light at night, propranolol administration or superior cervical ganglionectomy. J Neural Transm 54:275–284
Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C (2000) Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol 526:695–702
Rea MS, Bullough JD, Figueiro MG (2001) Human melatonin suppression by light: a case for scotopic efficiency. Neurosci Lett 299:45–48
Rea MS, Bullough JD, Figueiro MG (2002) Phototransduction for human melatonin suppression. J Pineal Res 32:209–213
Cardinali DP, Larin F, Wurtman RJ (1972) Control of the rat pineal gland by light spectra. Proc Natl Acad Sci USA 69:2003–2005
Brainard GC, Richardson BA, King TS, Reiter RJ (1984) The influence of different light spectra on the suppression of pineal melatonin content in the Syrian hamster. Brain Res 294:333– 339
Nelson DE, Takahashi JS (1991) Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Res 554:272–277
Lucas RJ, Freedman MS, Munoz M, Garcia-Fernandez JM, Foster RG (1999) Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science 284:505–507
Benshoff HM, Brainard GC, Rollag MD, Lynch GR (1987) Suppression of pineal melatonin in Peromyscus leucopus by different monochromatic wavelengths of visible and near-ultraviolet light (UV-A). Brain Res 420:397–402
Podolin PL, Rollag MD, Brainard GC (1987) The suppression of nocturnal pineal melatonin in the Syrian hamster: dose-response curves at 500 and 360 nm. Endocrinology 121:266–270
Brainard GC, Barker FM, Hoffman RJ, Stetson MH, Hanifin JP, Podolin PL, Rollag MD (1994) Ultraviolet regulation of neuroendocrine and circadian physiology in rodents. Vision Res 34:1521–1533
Brainard GC, Hanifin JP, Greeson JM etal. (2001) Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci 21:6405–6412
Thapan K, Arendt J, Skene DJ (2001) An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol 535:261–267
Sharma VK, Chandrashekaran MK, Singaravel M, Subbaraj R (1999) Relationship between light intensity and phase resetting in a mammalian circadian system. J Exp Zool 283:181–185
Boivin DB, Duffy JF, Kronauer RE, Czeisler CA (1996) Dose-response relationships for resetting of human circadian clock by light. Nature 379:540–542
Provencio I, Foster RG (1995) Circadian rhythms in mice can be regulated by photoreceptors with cone-like characteristics. Brain Res 694:183–190
Yoshimura T, Ebihara S (1996) Spectral sensitivity of photoreceptors mediating phase-shifts of circadian rhythms in retinally degenerate CBA/J (rd/rd) and normal CBA/N (+/+) mice. J Comp Physiol A 178:797–802
Geetha L, Subbaraj R (1996) Green light evokes maximum phase shifts in the locomotor activity rhythm of the field mouse Mus booduga. J Photochem Photobiol B 33:79–82
McGuire RA, Rand WM, Wurtman RJ (1973) Entrainment of the body temperature rhythm in rats: effect of color and intensity of environmental light. Science 181:956–957
Takahashi JS, DeCoursey PJ, Bauman L, Menaker M (1984) Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature 308:186–188
Boulos Z (1995) Wavelength dependence of light-induced phase shifts and period changes in hamsters. Physiol Behav 57:1025–1033
Amir S, Robinson B (1995) Ultraviolet light entrains rodent suprachiasmatic nucleus pacemaker. Neuroscience 69:1005–1011
Freedman MS, Lucas RJ, Soni B etal. (1999) Regulation of mammalian circadian behavior by non-rod, non-cone, ocular photoreceptors. Science 284:502–504
Wright HR, Lack LC (2001) Effect of light wavelength on suppression and phase delay of the melatonin rhythm. Chronobiol Int 18:801–808
Wright HR, Lack LC, Kennaway DJ (2004) Differential effects of light wavelength in phase advancing the melatonin rhythm. J Pineal Res 36:140–144
Lockley SW, Brainard GC, Czeisler CA (2003) High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab 88:4502–4505
Warman VL, Dijk DJ, Warman GR, Arendt J, Skene DJ (2003) Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett 342:37–40
Revell VL, Arendt J, Terman M, Skene DJ (2005) Short-wavelength sensitivity of the human circadian system to phase-advancing light. J Biol Rhythms 20:270–272
Bierman A, Klein TR, Rea MS (2005) The Daysimeter: a device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol 16:2292–2299
Rea MS (ed.) (2000) IESNA Lighting Handbook: Reference and Application, 9th edn, Illuminating Engineering Society of North America, New York
Sekuler R, Blake R (1994) Perception, 3rd edn, New York, McGraw-Hill
Rapp LM, Williams TP (1980) The role of ocular pigmentation in protecting against retinal light damage. Vision Res 20:1127–1131
He Y, Rea MS, Bierman A, Bullough J (1997) Evaluating light source efficacy under mesopic conditions using reaction times. J Illum Eng Soc 26, 125–138
Wang L, El Azazi M, Eklund A, Lillemor W (2001) Background light adaptation of the retinal neuronal adaptive system: I. effect of background light intensity. Doc Ophthalmol 103:13–26
Lyubarsky AL, Daniele LL, Pugh EN (2004) From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Res 44:3235–3251
Rea MS, Figueiro MG, Bullough JD (2002) Circadian photobiology: An emerging framework for lighting practice and research. Lighting Res Technol 34:177–190
Acknowledgement
Preparation of this manuscript was supported by the Lighting Research Center and by the National Institute of Environmental Health Sciences grant ES11659.
Author information
Authors and Affiliations
Corresponding author
Additional information
The title is a variation on the title of John Steinbeck’s classic novel, Of Mice and Men.
Rights and permissions
About this article
Cite this article
Bullough, J.D., Rea, M.S. & Figueiro, M.G. Of Mice and Women: Light as a Circadian Stimulus in Breast Cancer Research. Cancer Causes Control 17, 375–383 (2006). https://doi.org/10.1007/s10552-005-0574-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10552-005-0574-1