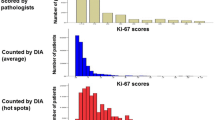
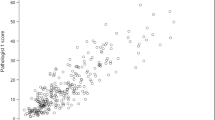
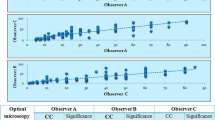
Abstract
Purpose
Ki-67 expression levels in breast cancer have prognostic and predictive significance. Therefore, accurate Ki-67 evaluation is important for optimal patient care. Although an algorithm developed by the International Ki-67 in Breast Cancer Working Group (IKWG) improves interobserver variability, it is tedious and time-consuming. In this study, we simplify IKWG algorithm and evaluate its interobserver agreement among breast pathologists in Ki-67 evaluation.
Methods
Six subspecialized breast pathologists (4 juniors, 2 seniors) assessed the percentage of positive cells in 5% increments in 57 immunostained Ki-67 slides. The time spent on each slide was recorded. Two rounds of ring study (R1, R2) were performed before and after training with the modified IKWG algorithm (eyeballing method at 400× instead of counting 100 tumor nuclei per area). Concordance was assessed using Kendall’s and Kappa coefficients.
Results
Analysis of ordinal scale ratings for all categories with 5% increments showed almost perfect agreement in R1 (0.821) and substantial in R2 (0.793); Seniors and juniors had substantial agreement in R1 (0.718 vs. 0.649) and R2 (0.756 vs. 0.658). In dichotomous scale analysis using 20% as the cutoff, the overall agreement was moderate in R1 (0.437) and R2 (0.479), among seniors (R1: 0.436; R2: 0.437) and juniors (R1: 0.445; R2: 0.505). Average scoring time per case was higher in R2 (71 vs. 37 s).
Conclusion
The modified IKWG algorithm does not significantly improve interobserver agreement. A better algorithm or assistance from digital image analysis is needed to improve interobserver variability in Ki-67 evaluation.



Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors
References
Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133(4):1710–1715
Zhu X, Chen L, Huang B, Wang Y, Ji L, Wu J, Di G, Liu G, Yu K, Shao Z et al (2020) The prognostic and predictive potential of Ki-67 in triple-negative Breast cancer. Sci Rep 10(1):225
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in Breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–72
Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P et al (2014) Recommendations for human epidermal growth factor receptor 2 testing in Breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med 138(2):241–256
Nielsen TO, Leung SCY, Rimm DL, Dodson A, Acs B, Badve S, Denkert C, Ellis MJ, Fineberg S, Flowers M et al (2021) Assessment of Ki67 in Breast Cancer: updated recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 113(7):808–819
Untch M, Gerber B, Harbeck N, Jackisch C, Marschner N, Mobus V, von Minckwitz G, Loibl S, Beckmann MW, Blohmer JU et al (2013) : 13th st. Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a German team of experts (Zurich 2013). Breast Care (Basel) 8(3):221–229
Davey MG, Ryan EJ, Abd Elwahab S, Elliott JA, McAnena PF, Sweeney KJ, Malone CM, McLaughlin R, Barry MK, Keane MM et al (2021) Clinicopathological correlates, oncological impact, and validation of Oncotype DX in a European Tertiary Referral Centre. Breast J 27(6):521–528
De Schutter H, Van Damme N, Colpaert C, Galant C, Lambein K, Cornelis A, Neven P, Van Eycken E (2015) Quality of pathology reporting is crucial for cancer care and registration: a baseline assessment for breast cancers diagnosed in Belgium in 2008. Breast 24(2):143–152
Abemaciclib for breast cancer (2020) Aust Prescr 43(3):94–95
Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW, Hilton JF et al (2016) Efficacy and safety of Abemaciclib, an inhibitor of CDK4 and CDK6, for patients with Breast Cancer, Non-small Cell Lung Cancer, and other solid tumors. Cancer Discov 6(7):740–753
Sledge GW Jr., Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA et al (2017) MONARCH 2: Abemaciclib in combination with Fulvestrant in Women with HR+/HER2− advanced Breast Cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Tredan O, Chen SC, Manso L et al (2017) MONARCH 3: Abemaciclib as initial therapy for advanced Breast Cancer. J Clin Oncol 35(32):3638–3646
Harbeck N, Rastogi P, Martin M, Tolaney SM, Shao ZM, Fasching PA, Huang CS, Jaliffe GG, Tryakin A, Goetz MP et al (2021) Adjuvant abemaciclib combined with endocrine therapy for high-risk early Breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol 32(12):1571–1581
Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, Viale G, Zabaglo LA, Penault-Llorca F, Bartlett JM et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906
Polley MY, Leung SC, Gao D, Mastropasqua MG, Zabaglo LA, Bartlett JM, McShane LM, Enos RA, Badve SS, Bane AL et al (2015) An international study to increase concordance in Ki67 scoring. Mod Pathol 28(6):778–786
Ellis MJ, Suman VJ, Hoog J, Goncalves R, Sanati S, Creighton CJ, DeSchryver K, Crouch E, Brink A, Watson M et al (2017) Ki67 Proliferation Index as a Tool for Chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of Breast Cancer: results from the American College of Surgeons Oncology Group Z1031 Trial (Alliance). J Clin Oncol 35(10):1061–1069
Giordano SH, Freedman RA, Somerfield MR, Optimal Adjuvant C (2022) Targeted Therapy Guideline Expert P: Abemaciclib with Endocrine Therapy in the treatment of high-risk early Breast Cancer: ASCO Optimal Adjuvant Chemotherapy and targeted Therapy Guideline Rapid Recommendation Update. J Clin Oncol 40(3):307–309
Dowsett MNT, Rimm DL, Hayes DF (2022) International Ki67 in Breast Cancer Working Group. Ki67 as a Companion Diagnostic: good or Bad News? J Clin Oncol 40(33):3796–3799
FDA axes diagnostic requirement for Lilly’ s Verzenio + endocrine therapy in certain breast cancer patients. https://endpts.com/fda-axes-diagnostic-requirement-for-lillys-verzenio-endocrine-therapy-in-certain-breast-cancer-patients/
Hida AI, Oshiro Y, Inoue H, Kawaguchi H, Yamashita N, Moriya T (2015) Visual assessment of Ki67 at a glance is an easy method to exclude many luminal-type breast cancers from counting 1000 cells. Breast Cancer 22(2):129–134
Shui R, Yu B, Bi R, Yang F, Yang W (2015) An interobserver reproducibility analysis of Ki67 visual assessment in Breast cancer. PLoS ONE 10(5):e0125131
Dowsett M, Nielsen TO, A’Hern R, Bartlett J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T et al (2011) Assessment of Ki67 in Breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664
Leung SCY, Nielsen TO, Zabaglo LA, Arun I, Badve SS, Bane AL, Bartlett JMS, Borgquist S, Chang MC, Dodson A et al (2019) Analytical validation of a standardised scoring protocol for Ki67 immunohistochemistry on Breast cancer excision whole sections: an international multicentre collaboration. Histopathology 75(2):225–235
Rimm DL, Leung SCY, McShane LM, Bai Y, Bane AL, Bartlett JMS, Bayani J, Chang MC, Dean M, Denkert C et al (2019) An international multicenter study to evaluate reproducibility of automated scoring for assessment of Ki67 in Breast cancer. Mod Pathol 32(1):59–69
Acs B, Pelekanou V, Bai Y, Martinez-Morilla S, Toki M, Leung SCY, Nielsen TO, Rimm DL (2019) Ki67 reproducibility using digital image analysis: an inter-platform and inter-operator study. Lab Invest 99(1):107–117
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
One author (DA) has worked as a consultant to PathAI. Other authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ai, D., Turashvili, G., Gjeorgjievski, S.G. et al. Subspecialized breast pathologists have suboptimal interobserver agreement in Ki-67 evaluation using 20% as the cutoff. Breast Cancer Res Treat 204, 415–422 (2024). https://doi.org/10.1007/s10549-023-07197-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07197-3