Abstract
Purpose
The role of neoadjuvant chemotherapy (NAC) in node-positive (N+) ER+/HER2- breast cancer (BC) is debated, given low total pathologic complete response (pCR) rates. However, the rate and impact of nodal pCR is unknown. We sought to evaluate nodal pCR rates and the impact on overall survival (OS). Further, we sought to validate the association between nodal pCR with age and Ki67.
Methods
We queried the National Cancer Database for cN + ER+/HER2- BC patients treated with NAC and surgery. Data from 2010 to 2018 were used to evaluate nodal pCR and OS, with multivariable Cox proportional hazards modeling for OS, as well as Ki67 for the years 2018–2019.
Results
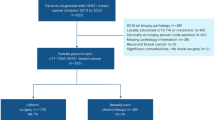
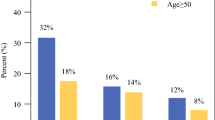
From 2010 to 2018, we identified 19,611 cN + ER+/HER2- BC patients treated with NAC. While total pCR occurred in only 7.4%, nodal pCR rates were nearly double (14.3%). Nodal pCR (+/- breast pCR) was seen in 21.7% and associated with 5-year OS rate of 86.1% (95% CI: 84.9–87.4%) versus 77.1% (95% CI: 76.3–77.9%) in patients without nodal pCR (p < 0.001). On multivariable analysis, nodal pCR had better OS (adjusted HR 0.57, 95% CI 0.52–0.63, p < 0.001) across all age groups. Of 2,444 patients with available Ki67, those with age < 50 and Ki67 ≥ 20% had the highest nodal pCR at 31.6%.
Conclusion
In cN + ER+/HER2- BC treated with NAC, nodal pCR is common, associated with age and Ki67, and prognostic for OS. These data strongly suggest that for cN + patients, eradication of nodal disease is critical for OS, and total pCR may not be the optimal measure of NAC benefit.


Similar content being viewed by others
Data Availability
The datasets analysed during the current study are available in the National Cancer Database repository and available to Commission on Cancer sites by application to the NCDB.
References
Giaquinto AN, Sung H, Miller KD et al (2022) Breast cancer statistics, 2022. CA Cancer J Clin 72(6):524–541. https://doi.org/10.3322/caac.21754
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Breast Cancer. Accessed 12/10/2022 (2022) https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
Early breast cancer trialists’ collaborative group (2005) effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717. https://doi.org/10.1016/S0140-6736(05)66544-0
Kalinsky K, Barlow WE, Gralow JR et al (2021) 21-gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 385:2336–2347. https://doi.org/10.1056/NEJMoa2108873
Sparano JA, Gray RJ, Makower DF et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379:111–121. https://doi.org/10.1056/NEJMoa1804710
Fisher B, Brown A, Mamounas E et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15(7):2483–2493. https://doi.org/10.1200/JCO.1997.15.7.2483
Caudle AS, Yang WT, Krishnamurthy S et al (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34:1072–1078. https://doi.org/10.1200/JCO.2015.64.0094
Boileau J-F, Poirier B, Basik M et al (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 33:258–264. https://doi.org/10.1200/JCO.2014.55.7827
Boughey JC, Suman VJ, Mittendorf EA et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310:1455–1461. https://doi.org/10.1001/jama.2013.278932
Yau C, Osdoit M, van der Noordaa M et al (2022) Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol 23:149–160. https://doi.org/10.1016/S1470-2045(21)00589-1
Boughey JC, McCall LM, Ballman KV et al (2014) Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (alliance) prospective multicenter clinical trial. Ann Surg 260:608–14 discussion 614–616. https://doi.org/10.1097/SLA.0000000000000924
Cortazar P, Zhang L, Untch M et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384:164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Whitworth PW, Beitsch PD, Pellicane JV et al (2022) Distinct neoadjuvant chemotherapy response and 5-year outcome in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast tumors that reclassify as basal-type by the 80-gene signature. JCO Precis Oncol 6:e2100463. https://doi.org/10.1200/PO.21.00463
Gluz O, Kuemmel S, Nitz U et al (2023) Nab-paclitaxel weekly versus dose-dense solvent-based paclitaxel followed by dose-dense epirubicin plus cyclophosphamide in high-risk HR+/HER2- early breast cancer: results from the neoadjuvant part of the WSG-ADAPT-HR+/HER2- trial. Ann Oncol 34:531–542. https://doi.org/10.1016/j.annonc.2023.04.002
Petruolo OA, Pilewskie M, Patil S et al (2017) Standard pathologic features can be used to identify a subset of estrogen receptor-positive, HER2 negative patients likely to benefit from neoadjuvant chemotherapy. Ann Surg Oncol 24:2556–62. https://doi.org/10.1245/s10434-017-5898-z
Boughey JC, Hoskin TL, Goetz MP (2022) Neoadjuvant chemotherapy and nodal response rates in luminal breast cancer: effects of age and tumor Ki67. Ann Surg Oncol 29:5747–5756. https://doi.org/10.1245/s10434-022-11871-z
Kuehn T, Bauerfeind I, Fehm T et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14:609–618. https://doi.org/10.1016/S1470-2045(13)70166-9
Korde LA, Somerfield MR, Carey LA et al (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39:1485–1505. https://doi.org/10.1200/JCO.20.03399
Schmid P, Cortes J, Pusztai L et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810–821. https://doi.org/10.1056/NEJMoa1910549
Laws A, Pastorello R, Dey T et al (2022) Impact of the histologic pattern of residual tumor after neoadjuvant chemotherapy on recurrence and survival in stage I-III breast cancer. Ann Surg Oncol 29:7726–7736. https://doi.org/10.1245/s10434-022-12054-6
Wong SM, Almana N, Choi J et al (2019) Prognostic significance of residual axillary nodal micrometastases and isolated tumor cells after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol 26:3502–3509. https://doi.org/10.1245/s10434-019-07517-2
Piccart M, van ‘t Veer LJ, Poncet C et al (2021) 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol 22:476–488. https://doi.org/10.1016/S1470-2045(21)00007-3
Thomssen C, Balic M, Harbeck N, Gnant M (2021) St. Gallen/Vienna 2021: a brief summary of the consensus discussion on customizing therapies for women with early breast cancer. Breast Care (Basel) 16:135–143. https://doi.org/10.1159/000516114
Funding
This work was supported in part by the Mayo Clinic Breast Cancer Specialized Program of Research Excellence Grant, National Institutes of Health (P50CA 116201) (MPG) and the George M. Eisenberg Foundation for Charities (to MPG, JCB).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Judy Boughey, Tanya Hoskin, Courtney Day, Dan Moldoveanu and Matthew Goetz. The first draft of the manuscript was written by Dan Moldoveanu and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
Dr. Boughey receives research support paid to her institution from Eli Lilly and SymBioSis and is on a DSMB for CairnsSurgical. She has received honoraria for speaking for PER, PeerView, EndoMag and contributed a chapter to UpToDate. Dr. Goetz reports personal fees for CME activities from Research to Practice, Clinical Education Alliance, Medscape, and MJH Life Sciences; personal fees for serving as a panelist on a panel discussion from Total Health Conferencing and personal fees for serving as a moderator for Curio Science; consulting fees to Mayo Clinic from ARC Therapeutics, AstraZeneca, Biotheranostics, Blueprint Medicines, Lilly, RNA Diagnostics, Sanofi Genzyme, and Seattle Genetics; and grant funding to Mayo Clinic from Lilly, Pfizer, SymBioSis, Sermonix, Atossa, and AstraZeneca.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Presentation: This work was presented as a poster presentation at the San Antonio Breast Cancer Symposium on December 6–10, 2022, and as a poster presentation at the Society of Surgical Oncology Annual Meeting on March 22–25, 2023.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moldoveanu, D., Hoskin, T.L., Day, C.N. et al. Nodal pCR and overall survival following neoadjuvant chemotherapy for node positive ER+/Her2- breast cancer. Breast Cancer Res Treat 203, 419–428 (2024). https://doi.org/10.1007/s10549-023-07152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-07152-2