Abstract
Purpose
Phyllodes tumor of the breast is a kind of rare neoplasm, which accounts for less than 1% of all breast tumors. Malignant phyllodes tumor (MPT) is the highest risk subtype of phyllodes tumor, and is characterized by the tendency of local recurrence and distant metastasis. The prediction of prognosis and the individual therapy for MPT is still challenging. It’s urgent to develop a new reliable in vitro preclinical model in order to understand this disease better and to explore appropriate anticancer drugs for individual patients.
Methods
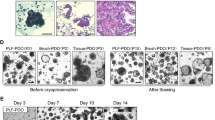
Two surgically resected MPT specimens were processed for organoid establishment. MPT organoids were subsequently subjected to H&E staining, immunohistochemical analysis and drug screening, respectively.
Results
We successfully established two organoid lines from different patients with MPT. The MPT organoids can well retain the histological features and capture the marker expression in original tumor tissues, including p63, vimentin, Bcl-2, CD34, c-Kit, and Ki-67, even after a long-term culture. The dose titration tests of eight typical chemotherapeutic drugs (paclitaxel, docetaxel, vincristine, doxorubicin, cisplatin, gemcitabine, cyclophosphamide, ifosfamide) on the two MPT organoid lines showed patient-specific drug responses and varying IC50 values. Of all the drugs, doxorubicin and gemcitabine showed the best anti-tumor effect on the two organoid lines.
Conclusion
Organoids derived from MPT may be a novel preclinical model for testing personalized therapies for patients with MPT.





Similar content being viewed by others
Data availability
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Consent to publish
All authors have seen and approved the manuscript and consent publication.
Abbreviations
- EGF:
-
Epidermal growth factor
- ER:
-
Estrogen receptor
- FBS:
-
Fetal bovine serum
- FGF-7:
-
Fibroblast growth factor 7
- FGF-10:
-
Fibroblast growth factor 10
- H&E:
-
Hematoxylin and eosin
- HER2:
-
Human epidermal growth factor receptor-2
- HRCT:
-
High resolution computed tomography
- IC50 :
-
Half-maximal inhibitory concentration
- IHC:
-
Immunohistochemical
- MPT:
-
Malignant phyllodes tumor of the breast
- MRI:
-
Magnetic resonance imaging
- PDXs:
-
Patient-derived xenografts
- PR:
-
Progesterone receptor
- ROCK:
-
Rho-associated protein kinase
- WHO:
-
World Health Organization
- 2D:
-
Two-dimensional
- 3D:
-
Three-dimensional
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Rosen PP, Oberman HA (1993) Tumors of the mammary gland. Armed Forces Institute of Pathology, Washington, DC, p 7
Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ (2012) World Health Organization classification of tumours. Lyon, IARC, p 4
Jorge Blanco A, Vargas Serrano B, Rodríguez Romero R, Martínez CE (1999) Phyllodes tumors of the breast. Eur Radiol 9(2):356–360. https://doi.org/10.1007/s003300050680
Tan PH, Thike AA, Tan WJ, Thu MM, Busmanis I, Li H et al (2012) Predicting clinical behaviour of breast phyllodes tumours: a nomogram based on histological criteria and surgical margins. J Clin Pathol 65(1):69–76. https://doi.org/10.1136/jclinpath-2011-200368
Lenhard MS, Kahlert S, Himsl I, Ditsch N, Untch M, Bauerfeind I (2008) Phyllodes tumour of the breast: clinical follow-up of 33 cases of this rare disease. Eur J Obstet Gynecol Reprod Biol 138(2):217–221. https://doi.org/10.1016/j.ejogrb.2007.08.002
Kapiris I, Nasiri N, A’Hern R, Healy V, Gui GP (2001) Outcome and predictive factors of local recurrence and distant metastases following primary surgical treatment of high-grade malignant phyllodes tumours of the breast. Eur J Surg Oncol 27(8):723–730. https://doi.org/10.1053/ejso.2001.1207
Zhang Y, Kleer CG (2016) Phyllodes tumor of the breast: histopathologic features, differential diagnosis, and molecular/genetic updates. Arch Pathol Lab Med 140(7):665–671. https://doi.org/10.5858/arpa.2016-0042-RA
Tse GM, Niu Y, Shi HJ (2010) Phyllodes tumor of the breast: an update. Breast Cancer 17(1):29–34. https://doi.org/10.1007/s12282-009-0114-z
Chaney AW, Pollack A, McNeese MD, Zagars GK, Pisters PW, Pollock RE et al (2000) Primary treatment of cystosarcoma phyllodes of the breast. Cancer 89(7):1502–1511. https://doi.org/10.1002/1097-0142(20001001)89:7%3c1502::aid-cncr13%3e3.0.co;2-p
Strode M, Khoury T, Mangieri C, Takabe K (2017) Update on the diagnosis and management of malignant phyllodes tumors of the breast. Breast 33:91–96. https://doi.org/10.1016/j.breast.2017.03.001
Domcke S, Sinha R, Levine DA, Sander C, Schultz N (2013) Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun 4:2126. https://doi.org/10.1038/ncomms3126
Kamb A (2005) What’s wrong with our cancer models? Nat Rev Drug Discov 4(2):161–165. https://doi.org/10.1038/nrd1635
Siolas D, Hannon GJ (2013) Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res 73(17):5315–5319. https://doi.org/10.1158/0008-5472.CAN-13-1069
Tuveson D, Clevers H (2019) Cancer modeling meets human organoid technology. Science 364(6444):952–955. https://doi.org/10.1126/science.aaw6985
Sachs N, de Ligt J, Kopper O, Gogola E, Bounova G, Weeber F et al (2018) A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172(1–2):373–386. https://doi.org/10.1016/j.cell.2017.11.010
Vlachogiannis G, Hedayat S, Vatsiou A, Jamin Y, Fernández-Mateos J, Khan K et al (2018) Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359(6378):920–926. https://doi.org/10.1126/science.aao2774
Tiriac H, Belleau P, Engle DD, Plenker D, Deschênes A, Somerville TDD et al (2018) Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discov 8(9):1112–1129. https://doi.org/10.1158/2159-8290.CD-18-0349
Yan HHN, Siu HC, Law S, Ho SL, Yue SSK, Tsui WY et al (2018) A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 23(6):882–897. https://doi.org/10.1016/j.stem.2018.09.016
Ooft SN, Weeber F, Dijkstra KK, McLean CM, Kaing S, van Werkhoven E et al (2019) Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci Transl Med 11(513):2574. https://doi.org/10.1126/scitranslmed.aay2574
Chen P, Zhang X, Ding R, Yang L, Lyu X, Zeng J et al (2021) Patient-derived organoids can guide personalized-therapies for patients with advanced breast cancer. Adv Sci 8(22):2101176. https://doi.org/10.1002/advs.202101176
Xu Y, Zhu X, Hahm HS, Wei W, Hao E, Hayek A et al (2010) Revealing a core signaling regulatory mechanism for pluripotent stem cell survival and self-renewal by small molecules. Proc Natl Acad Sci USA 107(18):8129–8134. https://doi.org/10.1073/pnas.1002024107
Chen D, Tan Y, Li Z, Li W, Yu L, Chen W et al (2021) Organoid cultures derived from patients with papillary thyroid cancer. J Clin Endocrinol Metab 106(5):1410–1426. https://doi.org/10.1210/clinem/dgab020
Cimino-Mathews A, Sharma R, Illei PB, Vang R, Argani P (2014) A subset of malignant phyllodes tumors express p63 and p40 a diagnostic pitfall in breast core needle biopsies. Am J Surg Pathol 38(12):1689–1696. https://doi.org/10.1097/PAS.0000000000000301
Thway K, Nicholson AG, Lawson K, Gonzalez D, Rice A, Balzer B et al (2011) Primary pulmonary myxoid sarcoma with EWSR1-CREB1 fusion: a new tumor entity. Am J Surg Pathol 35(11):1722–1732. https://doi.org/10.1097/PAS.0b013e318227e4d2
Virk RK, Khan A (2010) Pseudoangiomatous stromal hyperplasia: an overview. Arch Pathol Lab Med 134(7):1070–1074. https://doi.org/10.5858/2008-0686-RS.1
Moore T, Lee AH (2001) Expression of CD34 and bcl-2 in phyllodes tumours, fibroadenomas and spindle cell lesions of the breast. Histopathology 38(1):62–67. https://doi.org/10.1046/j.1365-2559.2001.01053.x
Miettinen M, Lasota J (2005) KIT (CD117): a review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol 13(3):205–220. https://doi.org/10.1097/01.pai.0000173054.83414.22
Wang F, Jia Y, Tong Z (2015) Comparison of the clinical and prognostic features of primary breast sarcomas and malignant phyllodes tumor. Jpn J Clin Oncol 45(2):146–152. https://doi.org/10.1093/jjco/hyu177
Pervaiz N, Colterjohn N, Farrokhyar F, Tozer R, Figueredo A, Ghert M (2008) A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 113(3):573–581. https://doi.org/10.1002/cncr.23592
Eilber FC, Brennan MF, Eilber FR, Eckardt JJ, Grobmyer SR, Riedel E et al (2007) Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg 246(1):105–113. https://doi.org/10.1097/01.sla.0000262787.88639.2b
Petrioli R, Coratti A, Correale P, D’Aniello C, Grimaldi L, Tanzini G et al (2002) Adjuvant epirubicin with or without ifosfamide for adult soft-tissue sarcoma. Am J Clin Oncol 25(5):468–473. https://doi.org/10.1097/00000421-200210000-00009
Kleer CG, Giordano TJ, Braun T, Oberman HA (2001) Pathologic, immunohistochemical, and molecular features of benign and malignant phyllodes tumors of the breast. Mod Pathol 14(3):185–190. https://doi.org/10.1038/modpathol.3880282
Ganesh K, Wu C, O’Rourke KP, Szeglin BC, Zheng Y, Sauvé CG et al (2019) A rectal cancer organoid platform to study individual responses to chemoradiation. Nat Med 25(10):1607–1614. https://doi.org/10.1038/s41591-019-0584-2
Yao Y, Xu X, Yang L, Zhu J, Wan J, Shen L (2020) Patient-derived organoids predict chemoradiation responses of locally advanced rectal cancer. Cell Stem Cell 26(1):17–26. https://doi.org/10.1016/j.stem.2019.10.010
Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T et al (2013) Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499(7459):481–484. https://doi.org/10.1038/nature12271
Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y et al (2015) Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16(5):556–565. https://doi.org/10.1016/j.stem.2015.03.004
Bar-Ephraim YE, Kretzschmar K, Clevers H (2020) Organoids in immunological research. Nat Rev Immunol 20(5):279–293. https://doi.org/10.1038/s41577-019-0248-y
Dijkstra KK, Cattaneo CM, Weeber F, Chalabi M, van de Haar J, Fanchi LF et al (2018) Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174(6):1586–1598. https://doi.org/10.1016/j.cell.2018.07.009
Schnalzger TE, de Groot MH, Zhang C, Mosa MH, Michels BE, Röder J et al (2019) 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J 38(12):100928. https://doi.org/10.15252/embj.2018100928
Neal JT, Li X, Zhu J, Giangarra V, Grzeskowiak CL, Ju J et al (2018) Organoid modeling of the tumor immune microenvironment. Cell 175(7):1972-1988.e16. https://doi.org/10.1016/j.cell.2018.11.021
Roulis M, Kaklamanos A, Schernthanner M, Bielecki P, Zhao J, Kaffe E et al (2020) Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nature 580(7804):524–529. https://doi.org/10.1038/s41586-020-2166-3
Corrò C, Novellasdemunt L, Li VSW (2020) A brief history of organoids. Am J Physiol Cell Physiol 319(1):151–165. https://doi.org/10.1152/ajpcell.00120.2020
Kalbasi A, Ribas A (2020) Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol 20(1):25–39. https://doi.org/10.1038/s41577-019-0218-4
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8(9):1069–1086. https://doi.org/10.1158/2159-8290.CD-18-0367
Junttila MR, de Sauvage FJ (2013) Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 501(7467):346–354. https://doi.org/10.1038/nature12626
Klemm F, Joyce JA (2015) Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol 25(4):198–213. https://doi.org/10.1016/j.tcb.2014.11.006
Palucka AK, Coussens LM (2016) The basis of oncoimmunology. Cell 164(6):1233–1247. https://doi.org/10.1016/j.cell.2016.01.049
Aboulkheyr Es H, Montazeri L, Aref AR, Vosough M, Baharvand H (2018) Personalized cancer medicine: an organoid approach. Trends Biotechnol 36(4):358–371. https://doi.org/10.1016/j.tibtech.2017.12.005
Funding
This work was supported by the National Key R&D Program of China (2019YFA0906000), the Guangdong Basic and Applied Basic Research Foundation (2021A1515110618, 2022A1515011428), the Shenzhen Science and Technology Program (KCXFZ20211020163407011, JCYJ20210324105612034, JCYJ20220531094206014, GJHZ20180928115030292), the Shenzhen San-Ming Project (SZSM201612010), the Shenzhen Key Medical Discipline Construction Fund (SZXK017), and the Scientific Research Foundation of PEKING UNIVERSITY SHENZHEN HOSPITAL (KYQD2023251).
Author information
Authors and Affiliations
Contributions
XC, MW, DC, and JH conceived the study and designed experiments; XC, YF, XW, HW, YX, and JH recruited patients, performed operations, and collected and curated clinical annotation data; XC, MW, and DC performed the experiments; all authors were involved in data analysis and interpretation of the results; XC, DC, JY, and JH wrote the manuscript. All authors reviewed and gave final approval.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was approved by the Human Ethical Committee of Peking University Shenzhen Hospital (Approval No. 2019-062), and was carried out in accordance with the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chu, X., Wu, M., Yang, J. et al. Organoid models derived from patients with malignant phyllodes tumor of the breast. Breast Cancer Res Treat 200, 193–201 (2023). https://doi.org/10.1007/s10549-023-06973-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06973-5