Abstract
Purpose
Breast cancer has become the leading cause of cancer mortality in women. Although immune checkpoint inhibitors targeting programmed death-1 (PD-1) are promising, it remains unclear whether PD-L1 expression on circulating tumor cells (CTCs) has predictive and prognostic values in predicting and stratifying metastatic breast cancer (MBC) patients who can benefit from anti-PD-1 immunotherapy.
Methods
Twenty six MBC patients that received anti-PD-1 immunotherapy were enrolled in this study. The peptide-based Pep@MNPs method was used to isolate and enumerate CTCs from 2.0 ml of peripheral venous blood. The expression of PD-L1 on CTCs was evaluated by an established immunoscoring system categorizing into four classes (negative, low, medium, and high).
Results
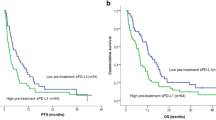
Our data showed that 92.3% (24/26) of patients had CTCs, 83.3% (20/26) of patients had PD-L1-positive CTCs, and 65.4% (17/26) of patients had PD-L1-high CTCs. We revealed that the clinical benefit rate (CBR) of patients with a cut-off value of ≥ 35% PD-L1-high CTCs (66.6%) was higher than the others (29.4%). We indicated that PD-L1 expression on CTCs from MBC patients treated with anti-PD-1 monotherapy was dynamic. We demonstrated that MBC patients with a cut-off value of ≥ 35% PD-L1-high CTCs had longer PFS (P = 0.033) and OS (P = 0.00058) compared with patients with a cut-off value of < 35% PD-L1-high CTCs.
Conclusion
Our findings suggested that PD-L1 expression on CTCs could predict the therapeutic response and clinical outcomes, providing a valuable predictive and prognostic biomarker for patients treated with anti-PD-1 immunotherapy.





Similar content being viewed by others
Data availability
All data are available within the manuscript or may be obtained from the corresponding authors upon reasonable request.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics. CA Cancer J Clin 72(1):7–33. https://doi.org/10.3322/caac.21708
Zou Y, Zou X, Zheng S, Tang H, Zhang L, Liu P, Xie X (2020) Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis. Ther Adv Med Oncol 12:1758835920940928. https://doi.org/10.1177/1758835920940928
Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, Peto R, Pritchard KI, Bergh J, Dowsett M, Hayes DF (2017) 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 377(19):1836–1846. https://doi.org/10.1056/NEJMoa1701830
Salvo EM, Ramirez AO, Cueto J, Law EH, Situ A, Cameron C, Samjoo IA (2021) Risk of recurrence among patients with HR-positive, HER2-negative, early breast cancer receiving adjuvant endocrine therapy: a systematic review and meta-analysis. Breast 57:5–17. https://doi.org/10.1016/j.breast.2021.02.009
Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, Minisini AM, Andreetta C, Mansutti M, Pisa FE, Fasola G, Puglisi F (2014) Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist 19(6):608–615. https://doi.org/10.1634/theoncologist.2014-0002
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA, Investigators IMT (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121. https://doi.org/10.1056/NEJMoa1809615
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA (2007) Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 13(15 Pt 1):4429–4434. https://doi.org/10.1158/1078-0432.CCR-06-3045
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321(3):288–300. https://doi.org/10.1001/jama.2018.19323
Barzaman K, Moradi-Kalbolandi S, Hosseinzadeh A, Kazemi MH, Khorramdelazad H, Safari E, Farahmand L (2021) Breast cancer immunotherapy: current and novel approaches. Int Immunopharmacol 98:107886. https://doi.org/10.1016/j.intimp.2021.107886
Chennamadhavuni A, Abushahin L, Jin N, Presley CJ, Manne A (2022) Risk factors and biomarkers for immune-related adverse events: a practical guide to identifying high-risk patients and rechallenging immune checkpoint inhibitors. Front Immunol 13:779691. https://doi.org/10.3389/fimmu.2022.779691
Gunturu KS, Pham TT, Shambhu S, Fisch MJ, Barron JJ, Debono D (2022) Immune checkpoint inhibitors: immune-related adverse events, healthcare utilization, and costs among commercial and Medicare Advantage patients. Support Care Cancer 30(5):4019–4026. https://doi.org/10.1007/s00520-022-06826-9
Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS, Hirsch FR (2021) PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol 18(6):345–362. https://doi.org/10.1038/s41571-021-00473-5
Hofman P, Heeke S, Alix-Panabieres C, Pantel K (2019) Liquid biopsy in the era of immuno-oncology: is it ready for prime-time use for cancer patients? Ann Oncol 30(9):1448–1459. https://doi.org/10.1093/annonc/mdz196
Boman C, Zerdes I, Martensson K, Bergh J, Foukakis T, Valachis A, Matikas A (2021) Discordance of PD-L1 status between primary and metastatic breast cancer: a systematic review and meta-analysis. Cancer Treat Rev 99:102257. https://doi.org/10.1016/j.ctrv.2021.102257
Wang Y, Zhou Y, Hu Z (2017) The functions of circulating tumor cells in early diagnosis and surveillance during cancer advancement. J Transl Int Med 5(3):135–138. https://doi.org/10.1515/jtim-2017-0029
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW, Hayes DF (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791. https://doi.org/10.1056/NEJMoa040766
Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, Doyle GV, Matera J, Allard WJ, Miller MC, Fritsche HA, Hortobagyi GN, Terstappen LW (2005) Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 23(7):1420–1430. https://doi.org/10.1200/JCO.2005.08.140
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224. https://doi.org/10.1158/1078-0432.CCR-05-2821
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW, Hayes DF (2006) Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–6409. https://doi.org/10.1158/1078-0432.CCR-05-1769
Liu Y, Liu Q, Wang T, Bian L, Zhang S, Hu H, Li S, Hu Z, Wu S, Liu B, Jiang Z (2013) Circulating tumor cells in HER2-positive metastatic breast cancer patients: a valuable prognostic and predictive biomarker. BMC Cancer 13:202. https://doi.org/10.1186/1471-2407-13-202
Jiang ZF, Cristofanilli M, Shao ZM, Tong ZS, Song EW, Wang XJ, Liao N, Hu XC, Liu Y, Wang Y, Zeng L, Zhang M (2013) Circulating tumor cells predict progression-free and overall survival in Chinese patients with metastatic breast cancer, HER2-positive or triple-negative (CBCSG004): a multicenter, double-blind, prospective trial. Ann Oncol 24(11):2766–2772. https://doi.org/10.1093/annonc/mdt246
Zhang S, Li L, Wang T, Bian L, Hu H, Xu C, Liu B, Liu Y, Cristofanilli M, Jiang Z (2016) Real-time HER2 status detected on circulating tumor cells predicts different outcomes of anti-HER2 therapy in histologically HER2-positive metastatic breast cancer patients. BMC Cancer 16:526. https://doi.org/10.1186/s12885-016-2578-5
Liu XR, Shao B, Peng JX, Li HP, Yang YL, Kong WY, Song GH, Jiang HF, Liang X, Yan Y (2017) Identification of high independent prognostic value of nanotechnology based circulating tumor cell enumeration in first-line chemotherapy for metastatic breast cancer patients. Breast 32:119–125. https://doi.org/10.1016/j.breast.2017.01.007
Liu X, Ran R, Shao B, Rugo HS, Yang Y, Hu Z, Wei Z, Wan F, Kong W, Song G, Jiang H, Liang X, Zhang R, Yan Y, Xu G, Li H (2018) Combined peripheral natural killer cell and circulating tumor cell enumeration enhance prognostic efficiency in patients with metastatic triple-negative breast cancer. Chin J Cancer Res 30(3):315–326. https://doi.org/10.21147/j.issn.1000-9604.2018.03.04
Zhou Y, Zhou J, Xiao J, Wang Y, Wang H, Shi H, Yue C, Jia F, Li P, Hu Z, Yang Y, Jiang Z, Wang T (2022) Prognostic relevance of estrogen receptor status in circulating tumor cells in breast cancer patients treated with endocrine therapy. Front Oncol 12:866293. https://doi.org/10.3389/fonc.2022.866293
Mazel M, Jacot W, Pantel K, Bartkowiak K, Topart D, Cayrefourcq L, Rossille D, Maudelonde T, Fest T, Alix-Panabieres C (2015) Frequent expression of PD-L1 on circulating breast cancer cells. Mol Oncol 9(9):1773–1782. https://doi.org/10.1016/j.molonc.2015.05.009
Jacot W, Mazel M, Mollevi C, Pouderoux S, D’Hondt V, Cayrefourcq L, Bourgier C, Boissiere-Michot F, Berrabah F, Lopez-Crapez E, Bidard FC, Viala M, Maudelonde T, Guiu S, Alix-Panabieres C (2020) Clinical correlations of programmed cell death ligand 1 status in liquid and standard biopsies in breast cancer. Clin Chem 66(8):1093–1101. https://doi.org/10.1093/clinchem/hvaa121
Papadaki MA, Koutsopoulos AV, Tsoulfas PG, Lagoudaki E, Aggouraki D, Monastirioti A, Koutoulaki C, Apostolopoulou CA, Merodoulaki AC, Papadaki C, Mavroudis D, Agelaki S (2020) Clinical relevance of immune checkpoints on circulating tumor cells in breast cancer. Cancers 12(2):376. https://doi.org/10.3390/cancers12020376
Darga EP, Dolce EM, Fang F, Kidwell KM, Gersch CL, Kregel S, Thomas DG, Gill A, Brown ME, Gross S, Connelly M, Holinstat M, Cobain EF, Rae JM, Hayes DF, Paoletti C (2021) PD-L1 expression on circulating tumor cells and platelets in patients with metastatic breast cancer. PLoS ONE 16(11):e0260124. https://doi.org/10.1371/journal.pone.0260124
Yue C, Jiang Y, Li P, Wang Y, Xue J, Li N, Li D, Wang R, Dang Y, Hu Z, Yang Y, Xu J (2018) Dynamic change of PD-L1 expression on circulating tumor cells in advanced solid tumor patients undergoing PD-1 blockade therapy. Oncoimmunology 7(7):e1438111. https://doi.org/10.1080/2162402X.2018.1438111
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu R, Zhang G, Zhao C, Zhang Y, Chen C, Wang Y, Yi X, Hu Z, Zou J, Wang Q (2019) Anti-PD-1 Antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res 25(2):515–523. https://doi.org/10.1158/1078-0432.CCR-18-2484
Tan Z, Yue C, Ji S, Zhao C, Jia R, Zhang Y, Liu R, Li D, Yu Q, Li P, Hu Z, Yang Y, Xu J (2021) Assessment of PD-L1 expression on circulating tumor cells for predicting clinical outcomes in patients with cancer receiving PD-1/PD-L1 blockade therapies. Oncologist 26(12):e2227–e2238. https://doi.org/10.1002/onco.13981
Marcus L, Fashoyin-Aje LA, Donoghue M, Yuan M, Rodriguez L, Gallagher PS, Philip R, Ghosh S, Theoret MR, Beaver JA, Pazdur R, Lemery SJ (2021) FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin Cancer Res 27(17):4685–4689. https://doi.org/10.1158/1078-0432.CCR-21-0327
O’Meara TA, Tolaney SM (2021) Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget 12(5):394–400. https://doi.org/10.18632/oncotarget.27877
Bai L, Du Y, Peng J, Liu Y, Wang Y, Yang Y, Wang C (2014) Peptide-based isolation of circulating tumor cells by magnetic nanoparticles. J Mater Chem B 2(26):4080–4088. https://doi.org/10.1039/c4tb00456f
Patel SP, Kurzrock R (2015) PD-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 14(4):847–856. https://doi.org/10.1158/1535-7163.MCT-14-0983
Meng X, Huang Z, Teng F, Xing L, Yu J (2015) Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev 41(10):868–876. https://doi.org/10.1016/j.ctrv.2015.11.001
Wang X, Teng F, Kong L, Yu J (2016) PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 9:5023–5039. https://doi.org/10.2147/OTT.S105862
Agostinetto E, Losurdo A, Nader-Marta G, Santoro A, Punie K, Barroso R, Popovic L, Solinas C, Kok M, de Azambuja E, Lambertini M (2022) Progress and pitfalls in the use of immunotherapy for patients with triple negative breast cancer. Expert Opin Investig Drugs 31(6):567–591. https://doi.org/10.1080/13543784.2022.2049232
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E, Iwata H, Masuda N, Otero MT, Gokmen E, Loi S, Guo Z, Zhao J, Aktan G, Karantza V, Schmid P, Investigators K (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396(10265):1817–1828. https://doi.org/10.1016/S0140-6736(20)32531-9
Schmid P, Cortes J, Pusztai L, McArthur H, Kummel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J, Investigators K (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. https://doi.org/10.1056/NEJMoa1910549
Rzhevskiy A, Kapitannikova A, Malinina P, Volovetsky A, Aboulkheyr Es H, Kulasinghe A, Thiery JP, Maslennikova A, Zvyagin AV, Ebrahimi Warkiani M (2021) Emerging role of circulating tumor cells in immunotherapy. Theranostics 11(16):8057–8075. https://doi.org/10.7150/thno.59677
Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W, Yu Z, Yang J, Wang B, Sun H, Xia H, Man Q, Zhong W, Antelo LF, Wu B, Xiong X, Liu X, Guan L, Li T, Liu S, Yang R, Lu Y, Dong L, McGettigan S, Somasundaram R, Radhakrishnan R, Mills G, Lu Y, Kim J, Chen YH, Dong H, Zhao Y, Karakousis GC, Mitchell TC, Schuchter LM, Herlyn M, Wherry EJ, Xu X, Guo W (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560(7718):382–386. https://doi.org/10.1038/s41586-018-0392-8
Lee JS, Magbanua MJM, Park JW (2016) Circulating tumor cells in breast cancer: applications in personalized medicine. Breast Cancer Res Treat 160(3):411–424. https://doi.org/10.1007/s10549-016-4014-6
Li W, Wang H, Zhao Z, Gao H, Liu C, Zhu L, Wang C, Yang Y (2019) Emerging nanotechnologies for liquid biopsy: the detection of circulating tumor cells and extracellular vesicles. Adv Mater 31(45):e1805344. https://doi.org/10.1002/adma.201805344
Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol 34(21):2460–2467. https://doi.org/10.1200/JCO.2015.64.8931
Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, Winer EP, Mukai H, Tamura K, Armstrong A, Liu MC, Iwata H, Ryvo L, Wimberger P, Rugo HS, Tan AR, Jia L, Ding Y, Karantza V, Schmid P (2019) Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30(3):405–411. https://doi.org/10.1093/annonc/mdy518
Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, Cescon DW, Iwata H, Campone M, Nanda R, Hui R, Curigliano G, Toppmeyer D, O’Shaughnessy J, Loi S, Paluch-Shimon S, Tan AR, Card D, Zhao J, Karantza V, Cortes J (2019) Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol 30(3):397–404. https://doi.org/10.1093/annonc/mdy517
Winer EP, Lipatov O, Im SA, Goncalves A, Munoz-Couselo E, Lee KS, Schmid P, Tamura K, Testa L, Witzel I, Ohtani S, Turner N, Zambelli S, Harbeck N, Andre F, Dent R, Zhou X, Karantza V, Mejia J, Cortes J, Investigators Group (2021) Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): a randomised, open-label, phase 3 trial. Lancet Oncol 22(4):499–511. https://doi.org/10.1016/S1470-2045(20)30754-3
Chumsri S, Sokol ES, Soyano-Muller AE, Parrondo RD, Reynolds GA, Nassar A, Thompson EA (2020) Durable complete response with immune checkpoint inhibitor in breast cancer with high tumor mutational burden and APOBEC signature. J Natl Compr Canc Netw 18(5):517–521. https://doi.org/10.6004/jnccn.2020.7543
Braso-Maristany F, Sanso M, Chic N, Martinez D, Gonzalez-Farre B, Sanfeliu E, Ghiglione L, Carcelero E, Garcia-Corbacho J, Sanchez M, Soy D, Jares P, Peg V, Saura C, Munoz M, Prat A, Vivancos A (2021) Case report: a case study documenting the activity of atezolizumab in a PD-L1-negative triple-negative breast cancer. Front Oncol 11:710596. https://doi.org/10.3389/fonc.2021.710596
Chic N, Braso-Maristany F, Prat A (2022) Biomarkers of immunotherapy response in breast cancer beyond PD-L1. Breast Cancer Res Treat 191(1):39–49. https://doi.org/10.1007/s10549-021-06421-2
Funding
This work was supported by grants from Research Foundation for Advanced Talents of Fujian Medical University (XRCZX2017020, XRCZX2019005), Beijing Natural Science Foundation (7192198), Strategic Priority Research Program of Chinese Academy of Sciences (XDB36000000, XDB38010400), the National Natural Science Foundation of China (32027801, 31870992, 21775031), the Natural Science Foundation of Fujian Province (2022J01203), CAS-JSPS (GJHZ2094), Science and Technology Service Network Initiative of the Chinese Academy of Sciences (KFJ-STS-ZDTP-079).
Author information
Authors and Affiliations
Contributions
TW, ZJ, YY, and ZH contributed to the study conception and design. Material preparation, data collection and analysis were performed by YZ, JZ, XH, HS, XL, and AW. The first draft of the manuscript was written by YZ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Consent to participate
Written informed consent was obtained from the parents.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Zhou, J., Hao, X. et al. Efficacy relevance of PD-L1 expression on circulating tumor cells in metastatic breast cancer patients treated with anti-PD-1 immunotherapy. Breast Cancer Res Treat 200, 281–291 (2023). https://doi.org/10.1007/s10549-023-06972-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06972-6