Abstract
Purpose
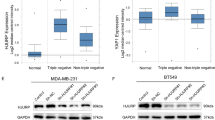
POU3F2 is associated with malignant behaviors and poor prognosis in cancer. However, the function and mechanism of POU3F2 in breast cancer remain to be elucidated. Our study aimed to explore the role of POU3F2 in triple-negative breast cancer and radiotherapy.
Methods
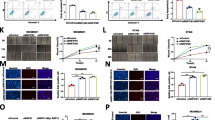
POU3F2 expression was examined by RT-PCR and Western blot. The proliferation of cancer cells was measured by MTT assay. Migration of cancer cells was determined by Transwell assay and wound healing assay. To determine which protein interacts with POU3F2, Co-IP was performed. Survival analysis was performed based on the online database GEPIA. DNA damage after radiation was examined by Comet Assay. Radiosensitivity was evaluated with clonogenic survival assays. A tumor xenograft model was established with MDA-MB-231 breast cancer cells in BALB/c nude mice to explore the effect of POU3F2 in vivo.
Results
We found that the expression of POU3F2 was significantly elevated in breast cancer cells, especially in TNBC, and higher POU3F2 expression was related to poor prognosis of patients with breast cancer. Functional assays revealed that POU3F2 promoted proliferation, migration, and invasion of triple-negative breast cancer (TNBC) cells in vitro and in vivo. In addition, the knockdown of POU3F2 decreased the radioresistance of TNBC cells in vitro. Furthermore, POU3F2 could enhance the activation of the Akt pathway by interacting with ARNT2, thereby promoting proliferation and radioresistance in TNBC cells.
Conclusions
Our results provide evidence that high expression of POU3F2 promotes radioresistance in triple-negative breast cancer via Akt pathway activation by interacting with ARNT2.




Similar content being viewed by others
Abbreviations
- TNBC:
-
Triple-negative breast cancer
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER:
-
Human epidermal growth factor receptor 2
- RT:
-
Radiotherapy
- ICI:
-
Immune checkpoint inhibitors
- TBP:
-
TATA-binding protein
- SCLC:
-
Small cell lung cancer
- NSCLC:
-
Non-small cell lung cancer
- ARNT2:
-
Aryl hydrocarbon receptor nuclear translocator 2
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. https://doi.org/10.3322/caac.21660
Foulkes WD, Smith IE, Reis-Filho JS (2010) Triple-negative breast cancer. N Engl J Med 363(20):1938–1948
Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, Godwin J et al (2011) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 378(9804):1707–1716
Ellmann L, Joshi MB, Resink TJ, Bosserhoff AK, Kuphal S (2012) BRN2 is a transcriptional repressor of CDH13 (T-cadherin) in melanoma cells. Lab Investig 92(12):1788–1800
Smit DJ, Smith AG, Parsons PG, Muscat GE, Sturm RA (2000) Domains of Brn-2 that mediate homodimerization and interaction with general and melanocytic transcription factors. Eur J Biochem 267(21):6413–6422
Fujii H, Hamada H (1993) A CNS-specific POU transcription factor, Brn-2, is required for establishing mammalian neural cell lineages. Neuron 11(6):1197–1206
Sakaeda M, Sato H, Ishii J, Miyata C, Kamma H, Shishido-Hara Y, Shimoyamada H, Fujiwara M, Endo T, Tanaka R et al (2013) Neural lineage-specific homeoprotein BRN2 is directly involved in TTF1 expression in small-cell lung cancer. Lab Investig 93(4):408–421
Ishii J, Sato H, Sakaeda M, Shishido-Hara Y, Hiramatsu C, Kamma H, Shimoyamada H, Fujiwara M, Endo T, Aoki I et al (2013) POU domain transcription factor BRN2 is crucial for expression of ASCL1, ND1 and neuroendocrine marker molecules and cell growth in small cell lung cancer. Pathol Int 63(3):158–168
Huang YH, Klingbeil O, He XY, Wu XS, Arun G, Lu B, Somerville T, Milazzo JP, Wilkinson JE, Demerdash OE et al (2018) POU2F3 is a master regulator of a tuft cell-like variant of small cell lung cancer. Genes Dev 32(13–14):915–928
Bishop JL, Thaper D, Vahid S, Davies A, Ketola K, Kuruma H, Jama R, Nip KM, Angeles A, Johnson F et al (2017) The master neural transcription factor BRN2 is an androgen receptor-suppressed driver of neuroendocrine differentiation in prostate cancer. Cancer Discov 7(1):54–71
Zhang Z, Zhou C, Li X, Barnes SD, Mu P (2020) Loss of CHD1 promotes heterogeneous mechanisms of resistance to AR-targeted therapy via chromatin dysregulation. Cancer Cell. https://doi.org/10.1016/j.ccell.2020.03.001
Herbert K, Binet R, Lambert JP, Louphrasitthiphol P, Kalkavan H, Sesma-Sanz L, Robles-Espinoza CD, Sarkar S, Suer E, Andrews S et al (2019) BRN2 suppresses apoptosis, reprograms DNA damage repair, and is associated with a high somatic mutation burden in melanoma. Genes Dev 33(5–6):310–332
Rahman MA, Liess L, Lellahi MS, Gjerde CH, Saed HS, Mutlu E, Zhu H, Wang J, Enger PØ (2015) Abstract 2101: the transcription factor POU3F2 is expressed in human gliomas and promotes tumorigenesis in vivo. Cancer Res (Chicago, Ill.) 75(15_Supplement):2101
Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee AV et al (2018) An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell 173(2):400–416
Zheng J, Wang B, Zheng R, Zhang J, Huang C, Zheng R, Huang Z, Qiu W, Liu M, Yang K et al (2020) Linc-RA1 inhibits autophagy and promotes radioresistance by preventing H2Bub1/USP44 combination in glioma cells. Cell Death Dis 11(9):758
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z (2017) GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 45(W1):W98–W102
Deorukhkar A, Krishnan S (2010) Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol 80(12):1904–1914
Bhat K, Balasubramaniyan V, Vaillant B, Ezhilarasan R, Hummelink K, Hollingsworth F, Wani K, Heathcock L, James JD, Goodman LD et al (2013) Mesenchymal differentiation mediated by NF-kappaB promotes radiation resistance in glioblastoma. Cancer Cell 24(3):331–346
Galeaz C, Totis C, Bisio A (2021) Radiation resistance: a matter of transcription factors. Front Oncol 11:662840
Baskar R, Dai J, Wenlong N, Yeo R, Yeoh KW (2014) Biological response of cancer cells to radiation treatment. Front Mol Biosci 1:24
Mah LJ, El-Osta A, Karagiannis TC (2010) GammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia 24(4):679–686
Guo Y, Zhu XD, Qu S, Li L, Su F, Li Y, Huang ST, Li DR (2012) Identification of genes involved in radioresistance of nasopharyngeal carcinoma by integrating gene ontology and protein-protein interaction networks. Int J Oncol 40(1):85–92
Yang B, Yang E, Liao H, Wang Z, Den Z, Ren H (2015) ARNT2 is downregulated and serves as a potential tumor suppressor gene in non-small cell lung cancer. Tumour Biol 36(3):2111–2119
Kimura Y, Kasamatsu A, Nakashima D, Yamatoji M, Minakawa Y, Koike K, Fushimi K, Higo M, Endo-Sakamoto Y, Shiiba M et al (2016) ARNT2 regulates tumoral growth in oral squamous cell carcinoma. J Cancer 7(6):702–710
Garrido-Castro AC, Lin NU, Polyak K (2019) Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov 9(2):176–198
Gupta GK, Collier AL, Lee D, Hoefer RA, Zheleva V, Siewertsz VRL, Tang-Tan AM, Guye ML, Chang DZ, Winston JS et al (2020) Perspectives on triple-negative breast cancer: current treatment strategies, Unmet needs, and potential targets for future therapies. Cancers (Basel). https://doi.org/10.3390/cancers12092392
Suva ML, Rheinbay E, Gillespie SM, Patel AP, Wakimoto H, Rabkin SD, Riggi N, Chi AS, Cahill DP, Nahed BV et al (2014) Reconstructing and reprogramming the tumor-propagating potential of glioblastoma stem-like cells. Cell 157(3):580–594
Bogeas A, Morvan-Dubois G, El-Habr EA, Lejeune FX, Defrance M, Narayanan A, Kuranda K, Burel-Vandenbos F, Sayd S, Delaunay V et al (2018) Changes in chromatin state reveal ARNT2 at a node of a tumorigenic transcription factor signature driving glioblastoma cell aggressiveness. Acta Neuropathol 135(2):267–283
Simmons JL, Pierce CJ, Al-Ejeh F, Boyle GM (2017) MITF and BRN2 contribute to metastatic growth after dissemination of melanoma. Sci Rep 7(1):10909
Martinez V, Kennedy S, Doolan P, Gammell P, Joyce H, Kenny E, Prakash MJ, Ryan E, O’Connor R, Crown J et al (2008) Drug metabolism-related genes as potential biomarkers: analysis of expression in normal and tumour breast tissue. Breast Cancer Res Treat 110(3):521–530
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11(5):329–341
Li HF, Kim JS, Waldman T (2009) Radiation-induced Akt activation modulates radioresistance in human glioblastoma cells. Radiat Oncol 4:43
Chang L, Graham PH, Hao J, Ni J, Bucci J, Cozzi PJ, Kearsley JH, Li Y (2014) PI3K/Akt/mTOR pathway inhibitors enhance radiosensitivity in radioresistant prostate cancer cells through inducing apoptosis, reducing autophagy, suppressing NHEJ and HR repair pathways. Cell Death Dis 5:e1437
Schuurbiers OC, Kaanders JH, van der Heijden HF, Dekhuijzen RP, Oyen WJ, Bussink J (2009) The PI3-K/AKT-pathway and radiation resistance mechanisms in non-small cell lung cancer. J Thorac Oncol 4(6):761–767
Chang L, Graham PH, Ni J, Hao J, Bucci J, Cozzi PJ, Li Y (2015) Targeting PI3K/Akt/mTOR signaling pathway in the treatment of prostate cancer radioresistance. Crit Rev Oncol Hematol 96(3):507–517
Jia Y, Hao S, Jin G, Li H, Ma X, Zheng Y, Xiao D, Wang Y (2019) Overexpression of ARNT2 is associated with decreased cell proliferation and better prognosis in gastric cancer. Mol Cell Biochem 450(1–2):97–103
Funding
This work was supported by the Natural Science Foundation of Guangdong (Grant No. 2021A1515011537).
Author information
Authors and Affiliations
Contributions
GX and ZG contributed to the study conception and design. Material preparation, data collection, and analysis were performed by HZ, JZ, YF, and JL, ZL, XL, XD, YS, TT. The first draft of the manuscript was written by HZ and all authors commented on previous versions of the manuscript. All authors approved the final manuscript for publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of Nanfang Hospital and obeyed the rules required by the Guide for the Care and Use of Laboratory Animals. Registration No. of the study: NFYY-2021-0831 (The review list of the animal ethic committee to the animal protocol has been uploaded).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Zheng, J., Fu, Y. et al. Overexpression of POU3F2 promotes radioresistance in triple-negative breast cancer via Akt pathway activation. Breast Cancer Res Treat 198, 437–446 (2023). https://doi.org/10.1007/s10549-023-06876-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-023-06876-5