Abstract
Purpose
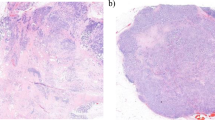
Tumor-stroma ratio (TSR) of invasive breast carcinoma has gained attention in recent years due to its prognostic significance. Previous studies showed TSR is a potential biomarker for indicating the tumor response to neoadjuvant chemotherapy. However, it is not clear how well TSR evaluation in biopsy specimens might reflect the TSR in resection specimens. We conducted a study to investigate whether biopsy evaluation of TSR can be an alternative method.
Method
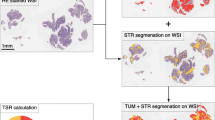
We collected cases with invasive breast carcinoma of no special type (IBC-NST) from University of Yamanashi hospital between 2011 and 2017 whose biopsy and resection specimens both had a pathologically diagnosis of IBC-NST (n = 146). We conceptualized a method for evaluating TSR in biopsy specimens within a preliminary cohort (n = 50). Within the studied cohort (n = 96), biopsy-based TSR (b-TSR) and resection-based TSR (r-TSR) were scored by two pathologists. We then evaluated our method’s validity and performance by measuring interobserver variability between the two pathologists, Spearman’s correlation between b-TSR and r-TSR, and the receiver operating characteristics (ROC) analysis for defining stroma-rich and stroma-poor tumors.
Results
Intra-class coefficient between the two pathologists was 0.59. The correlation coefficients between b-TSR and r-TSR in the two pathologists were 0.45 and 0.37. The ROC areas under the curve were 0.7 and 0.67. By considering an r-TSR of < 50% as stroma-rich, the sensitivity and specificity of detecting stroma-rich tumors were 64.1% and 66.7%, respectively, when b-TSR was < 40%.
Conclusion
Our current b-TSR evaluation method can provide information about r-TSR and facilitate pre-treatment therapy follow-up.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Vangangelt KMH, Green AR, Heemskerk IMF et al (2020) The prognostic value of the tumor-stroma ratio is most discriminative in patients with grade III or triple-negative breast cancer. Int J Cancer 146:2296–2304
Vangangelt KMH, Tollenaar LSA, van Pelt GW et al (2018) The prognostic value of tumor-stroma ratio in tumor-positive axillary lymph nodes of breast cancer patients. Int J Cancer 143:3194–3200
Xu Q, Yuan J-P, Chen Y-Y et al (2020) Prognostic significance of the tumor-stromal ratio in invasive breast cancer and a proposal of a new Ts-TNM staging system. J Oncol 2020:9050631
Hagenaars SC, de Groot S, Cohen D et al (2021) Tumor-stroma ratio is associated with Miller-Payne score and pathological response to neoadjuvant chemotherapy in HER2-negative early breast cancer. Int J Cancer 149:1181–1188
Mallya V, Singh V, Kaur N et al (2020) Does tumor stroma ratio of breast cancer trucut biopsy determine response to neoadjuvant therapy? Indian J Pathol Microbiol 63:S113–S116
Vangangelt KMH, van Pelt GW, Engels CC et al (2018) Prognostic value of tumor-stroma ratio combined with the immune status of tumors in invasive breast carcinoma. Breast Cancer Res Treat 168:601–612
Dekker TJA, van de Velde CJH, van Pelt GW et al (2013) Prognostic significance of the tumor-stroma ratio: validation study in node-negative premenopausal breast cancer patients from the EORTC perioperative chemotherapy (POP) trial (10854). Breast Cancer Res Treat 139:371–379
Wang M, He X, Chang Y et al (2017) A sensitivity and specificity comparison of fine needle aspiration cytology and core needle biopsy in evaluation of suspicious breast lesions: a systematic review and meta-analysis. Breast 31:157–166
Hagenaars SC, Vangangelt MHKiki et al (2022) Standardization of the tumor-stroma ratio scoring method for breast cancer research. Breast Cancer Res Treat 193(3):545–553
Koo TK, Li MY (2016) A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med 15:155–163
Odate T, Le M-K, Kawai M et al (2022) Tumor-infiltrating lymphocytes in breast FNA biopsy cytology: a predictor of tumor-infiltrating lymphocytes in histologic evaluation. Cancer Cytopathol 130(5):336–343
Akoglu H (2018) User’s guide to correlation coefficients. Turk J Emerg Med 18:91–93
Youden WJ (1950) Index for rating diagnostic tests. Cancer 3(1):32–35
Chung W, Eum HH, Lee H-O et al (2017) Single-cell RNA-seq enables comprehensive tumour and immune cell profiling in primary breast cancer. Nat Commun 8:15081
Roulot A, Héquet D, Guinebretière J-M et al (2016) Tumoral heterogeneity of breast cancer. Ann Biol Clin (Paris) 74:653–660
Bareche Y, Buisseret L, Gruosso T et al (2020) Unraveling triple-Negative breast cancer tumor microenvironment heterogeneity: towards an optimized treatment approach. J Natl Cancer Inst 112:708–719
Mesker WE, Junggeburt JMC, Szuhai K et al (2007) The carcinoma-stromal ratio of colon carcinoma is an independent factor for survival compared to lymph node status and tumor stage. Cell Oncol 29:387–398
Eriksen AC, Sørensen FB, Lindebjerg J et al (2018) The prognostic value of tumour stroma ratio and tumour budding in stage II colon cancer. a nationwide population-based study. Int J Colorectal Dis 33:1115–1124
Gao J, Shen Z, Deng Z et al (2021) Impact of tumor-stroma ratio on the prognosis of colorectal cancer: a systematic review. Front Oncol. 11:738080
Bever KM, Sugar EA, Bigelow E et al (2015) The prognostic value of stroma in pancreatic cancer in patients receiving adjuvant therapy. HPB (Oxford) 17:292–298
Wu J, Liang C, Chen M et al (2016) Association between tumor-stroma ratio and prognosis in solid tumor patients: a systematic review and meta-analysis. Oncotarget 7:68954–68965
Liu J, Li C, Huang K et al (2021) A classification based on tumor-stroma ratio and tumor budding for patients with muscle-invasive bladder cancer. Expert Rev Anticancer Ther 22(3):323–330
Park H, Lee Y, Lee H et al (2017) The prognostic significance of cancer-associated fibroblasts in pancreatic ductal adenocarcinoma. Tumour Biol 39:1010428317718403
Huang H, Brekken RA (2020) Recent advances in understanding cancer-associated fibroblasts in pancreatic cancer. Am J Physiol Cell Physiol 319:C233–C243
He R, Li D, Liu B et al (2021) The prognostic value of tumor-stromal ratio combined with TNM staging system in esophagus squamous cell carcinoma. J Cancer 12:1105–1114
Peng C, Liu J, Yang G et al (2018) The tumor-stromal ratio as a strong prognosticator for advanced gastric cancer patients: proposal of a new TSNM staging system. J Gastroenterol 53:606–617
Karpathiou G, Vieville M, Gavid M et al (2019) Prognostic significance of tumor budding, tumor-stroma ratio, cell nests size, and stroma type in laryngeal and pharyngeal squamous cell carcinomas. Head Neck 41:1918–1927
Hansen TF, Kjær-Frifeldt S, Lindebjerg J et al (2018) Tumor-stroma ratio predicts recurrence in patients with colon cancer treated with neoadjuvant chemotherapy. Acta Oncol 57:528–533
Bello IO, Wennerstrand PM, Suleymanova I et al (2021) Biopsy quality is essential for preoperative prognostication in oral tongue cancer. APMIS 129:118–127
Liang Y, Zhu Y, Lin H et al (2021) The value of the tumour-stroma ratio for predicting neoadjuvant chemoradiotherapy response in locally advanced rectal cancer: a case control study. BMC Cancer 21:729
Vangangelt KMH, Kramer CJH, Bastiaannet E et al (2020) The intra-tumoural stroma in patients with breast cancer increases with age. Breast Cancer Res Treat 179:37–45
Tamura N, Hasebe T, Okada N et al (2009) Tumor histology in lymph vessels and lymph nodes for the accurate prediction of outcome among breast cancer patients treated with neoadjuvant chemotherapy. Cancer Sci 100:1823–1833
Dekker TJA, Charehbili A, Smit VTHBM et al (2015) Disorganised stroma determined on pre-treatment breast cancer biopsies is associated with poor response to neoadjuvant chemotherapy: results from the NEOZOTAC trial. Mol Oncol 9:1120–1128
Du Y, Zhang R, Zargari A et al (2018) Classification of tumor epithelium and stroma by exploiting image features learned by deep convolutional neural networks. Ann Biomed Eng 46:1988–1999
Ko ES, Han B-K, Kim RB et al (2014) Apparent diffusion coefficient in estrogen receptor-positive invasive ductal breast carcinoma: correlations with tumor-stroma ratio. Radiology 271:30–37
Yamaguchi K, Hara Y, Kitano I et al (2019) Tumor-stromal ratio (TSR) of invasive breast cancer: correlation with multi-parametric breast MRI findings. Br J Radiol 92:20181032
Li Y, Wang Z, Chen F et al (2019) Intravoxel incoherent motion diffusion-weighted MRI in patients with breast cancer: correlation with tumor stroma characteristics. Eur J Radiol 120:108686
Le MK, Odate T, Vuong HG et al (2022) Clinical detection of “extremely low-risk” follicular thyroid carcinoma: a population-based study of 7304 patients. Laryngoscope Invest Otolaryngol 7:1235–1242
Le MK, Kawai M, Odate T et al (2022) Metastatic risk stratification of 2526 medullary thyroid carcinoma patients: a study based on surveillance, epidemiology, and end results database. Endocr Pathol 33(3):348–358
Cha YJ, Ahn SG, Bae SJ et al (2018) Comparison of tumor-infiltrating lymphocytes of breast cancer in core needle biopsies and resected specimens: a retrospective analysis. Breast Cancer Res Treat 171:295–302
Acknowledgements
We thank Dr. Pam Zaber for her works in English editing.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by M-KL and TO. The first draft of the manuscript was written by M-KL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the University of Yamanashi (Approval number: 2547, date of issue: 4th January 2022).
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Le, MK., Odate, T., Kawai, M. et al. Investigating the role of core needle biopsy in evaluating tumor-stroma ratio (TSR) of invasive breast cancer: a retrospective study. Breast Cancer Res Treat 197, 113–121 (2023). https://doi.org/10.1007/s10549-022-06768-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06768-0