Abstract
Purpose
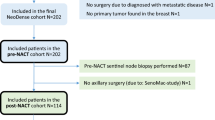
Routine axillary ultrasound (AxUS) in patients receiving neoadjuvant chemotherapy (NAC) remains controversial. Here, we report rates of AxUS-detected nodal disease among patients with normal clinical exams, and rates of pathologic nodal disease after NAC based on method of nodal disease detection.
Methods
Clinicopathologic findings were prospectively collected for stage I-III breast cancer patients selected for NAC. All patients had pre-treatment AxUS, suspicious nodes were biopsied. The following four patient cohorts were examined: patients with suspicious exam or AxUS but negative biopsy (Suspicious cN0); those with normal exam and normal AxUS (Not Suspicious cN0); those with normal exam but suspicious AxUS and positive biopsy (AxUS-detected cN1); and those with abnormal exam and positive biopsy (exam-detected cN1). Sentinel (SLN) and non-sentinel lymph nodes (non-SLN) were evaluated by immunohistochemistry; nodal metastases of any size were considered positive.
Results
500 patients were included. Of 310 patients with normal axillary exams, 160 had suspicious AxUS, 65 were biopsy-negative (Suspicious cN0) and 95/310 (30.6%) were biopsy-positive (AxUS-detected cN1). Of 190 with abnormal axillary exams, 166 were biopsy-proven node-positive (exam-detected cN1) and 24 were AxUS or biopsy-negative (Suspicious cN0). Rates of pathologic nodal disease were 20/150 (13.3%) among Not Suspicious cN0 patients, 12/89 (13.5%) among Suspicious cN0 (p = 0.97). Rates of residual nodal disease were 55/95 (57.9%) among AxUS-detected cN1 patients, 102/166 (61.4%) among exam-detected cN1 (p = 0.57).
Conclusion
AxUS detected nodal disease in 30.6% of patients with normal clinical exams selected for NAC. Rates of pathologic nodal disease were similar among AxUS-detected and exam-detected cN1 patients.

Similar content being viewed by others
Data availability
This data can be de-identified and provided for review/reproducibility, upon request.
References
Pilewskie M, Zabor EC, Mamtani A, Barrio AV, Stempel M, Morrow M (2017) The optimal treatment plan to avoid axillary lymph node dissection in early-stage breast cancer patients differs by surgical strategy and tumor subtype. Ann Surg Oncol 24(12):3527–3533. https://doi.org/10.1245/s10434-017-6016-y
Feng Y, Huang R, He Y, Lu A, Fan Z, Fan T, Qi M, Wang X, Cao W, Xie Y, Wang T, Li J, Ouyang T (2015) Efficacy of physical examination, ultrasound, and ultrasound combined with fine-needle aspiration for axilla staging of primary breast cancer. Breast Cancer Res Treat 149(3):761–765. https://doi.org/10.1007/s10549-015-3280-z
Pilewskie M, Jochelson M, Gooch JC, Patil S, Stempel M, Morrow M (2016) Is preoperative axillary imaging beneficial in identifying clinically node-negative patients requiring axillary lymph node dissection? J Am Coll Surg 222(2):138–145. https://doi.org/10.1016/j.jamcollsurg.2015.11.013
Horwood C, Ma N, Hayek J, Terando AM, Agnese DM, Grignol V (2020) Does use of axillary ultrasound in clinically node-negative patients receiving neo-adjuvant systemic therapy for breast cancer lead to surgical overtreatment? Breast J 26(2):120–124. https://doi.org/10.1111/tbj.13481
Tolaney SM, Guo H, Pernas S, Barry WT, Dillon DA, Ritterhouse L, Schneider BP, Shen F, Fuhrman K, Baltay M, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis MJ, Shapira I, Wolff AC, Carey LA, Overmoyer B, Partridge AH, Hudis CA, Krop IE, Burstein HJ, Winer EP (2019) Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 37(22):1868–1875. https://doi.org/10.1200/jco.19.00066
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821. https://doi.org/10.1056/NEJMoa1910549
Wang X, Chen L, Sun Y, Zhang B (2020) Evaluation of axillary lymph node metastasis burden by preoperative ultrasound in early-stage breast cancer with needle biopsy-proven metastasis. Clin Transl Oncol 22(4):468–473. https://doi.org/10.1007/s12094-019-02162-3
Weiss A, King C, Grossmith S, Portnow L, Raza S, Nakhlis F, Dominici L, Mittendorf EA, King TA (2020) Poster discussion PD4–06. How often does retrieval of a clipped lymph node change adjuvant therapy recommendations? A prospective consecutive patient cohort. San Antonio Breast Cancer Symposium (virtual)
Barrio AV, Mamtani A, Eaton A, Brennan S, Stempel M, Morrow M (2017) Is routine axillary imaging necessary in clinically node-negative patients undergoing neoadjuvant chemotherapy? Ann Surg Oncol 24(3):645–651. https://doi.org/10.1245/s10434-017-5765-y
Iwamoto N, Aruga T, Asami H, Horiguchi SI (2020) False-negative ultrasound-guided fine-needle aspiration of axillary lymph nodes in breast cancer patients. Cytopathology 31(5):463–467. https://doi.org/10.1111/cyt.12877
Kane G, Fleming C, Heneghan H, McCartan D, James P, Trueick R, Harrington L, Nally F, Quinn C, O’Doherty A, McNally S, Rothwell J, Evoy D, Geraghty J, McDermott E, Prichard R (2019) False-negative rate of ultrasound-guided fine-needle aspiration cytology for identifying axillary lymph node metastasis in breast cancer patients. Breast J 25(5):848–852. https://doi.org/10.1111/tbj.13402
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Kuerer HM, Bowling M, Flippo-Morton TS, Byrd DR, Ollila DW, Julian TB, McLaughlin SA, McCall L, Symmans WF, Le-Petross HT, Haffty BG, Buchholz TA, Nelson H, Hunt KK (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA 310(14):1455–1461. https://doi.org/10.1001/jama.2013.278932
Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, Arnaout A, Brackstone M, McCready DR, Karp SE, Trop I, Lisbona A, Wright FC, Younan RJ, Provencher L, Patocskai E, Omeroglu A, Robidoux A (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 33(3):258–264. https://doi.org/10.1200/jco.2014.55.7827
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, Bedrosian I, Hobbs BP, DeSnyder SM, Hwang RF, Adrada BE, Shaitelman SF, Chavez-MacGregor M, Smith BD, Candelaria RP, Babiera GV, Dogan BE, Santiago L, Hunt KK, Kuerer HM (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34(10):1072–1078. https://doi.org/10.1200/jco.2015.64.0094
Kalinsky K, Barlow WE, Gralow JR, Meric-Bernstam F, Albain KS, Hayes DF, Lin NU, Perez EA, Goldstein LJ, Chia SKL, Dhesy-Thind S, Rastogi P, Alba E, Delaloge S, Martin M, Kelly CM, Ruiz-Borrego M, Gil-Gil M, Arce-Salinas CH, Brain EGC, Lee ES, Pierga JY, Bermejo B, Ramos-Vazquez M, Jung KH, Ferrero JM, Schott AF, Shak S, Sharma P, Lew DL, Miao J, Tripathy D, Pusztai L, Hortobagyi GN (2021) 21-Gene assay to inform chemotherapy benefit in node-positive breast cancer. N Engl J Med 385(25):2336–2347. https://doi.org/10.1056/NEJMoa2108873
Pilewskie M, Mautner SK, Stempel M, Eaton A, Morrow M (2016) Does a positive axillary lymph node needle biopsy result predict the need for an axillary lymph node dissection in clinically node-negative breast cancer patients in the ACOSOG Z0011 era? Ann Surg Oncol 23(4):1123–1128. https://doi.org/10.1245/s10434-015-4944-y
Harris CK, Tran HT, Lee K, Mylander C, Pack D, Rosman M, Tafra L, Umbricht CB, Andrade R, Liang W, Jackson RS (2017) Positive ultrasound-guided lymph node needle biopsy in breast cancer may not mandate axillary lymph node dissection. Ann Surg Oncol 24(10):3004–3010. https://doi.org/10.1245/s10434-017-5935-y
Yoo TK, Kang BJ, Kim SH, Song BJ, Ahn J, Park WC, Chae BJ (2020) Axillary lymph node dissection is not obligatory in breast cancer patients with biopsy-proven axillary lymph node metastasis. Breast Cancer Res Treat 181(2):403–409. https://doi.org/10.1007/s10549-020-05636-z
Attieh M, Jamali F, Berjawi G, Saadeldine M, Boulos F (2019) Shortcomings of ultrasound-guided fine needle aspiration in the axillary management of women with breast cancer. World J Surg Oncol 17(1):208. https://doi.org/10.1186/s12957-019-1753-y
Moo TA, Edelweiss M, Hajiyeva S, Stempel M, Raiss M, Zabor EC, Barrio A, Morrow M (2018) Is low-volume disease in the sentinel node after neoadjuvant chemotherapy an indication for axillary dissection? Ann Surg Oncol 25(6):1488–1494. https://doi.org/10.1245/s10434-018-6429-2
Burstein HJ, Curigliano G, Thürlimann B, Weber WP, Poortmans P, Regan MM, Senn HJ, Winer EP, Gnant M (2021) Customizing local and systemic therapies for women with early breast cancer: the St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol 32(10):1216–1235. https://doi.org/10.1016/j.annonc.2021.06.023
A Randomized Phase III Trial Comparing Axillary Lymph Node Dissection to Axillary Radiation in Breast Cancer Patients (cT1-3 N1) Who Have Positive Sentinel Lymph Node Disease After Neoadjuvant Chemotherapy https://clinicaltrials.gov/ct2/show/NCT01901094.
Diepstraten SC, Sever AR, Buckens CF, Veldhuis WB, van Dalen T, van den Bosch MA, Mali WP, Verkooijen HM (2014) Value of preoperative ultrasound-guided axillary lymph node biopsy for preventing completion axillary lymph node dissection in breast cancer: a systematic review and meta-analysis. Ann Surg Oncol 21(1):51–59. https://doi.org/10.1245/s10434-013-3229-6
Hotton J, Salleron J, Henrot P, Buhler J, Leufflen L, Rauch P, Marchal F (2020) Pre-operative axillary ultrasound with fine-needle aspiration cytology performance and predictive factors of false negatives in axillary lymph node involvement in early breast cancer. Breast Cancer Res Treat 183(3):639–647. https://doi.org/10.1007/s10549-020-05830-z
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, Liedtke C, von Minckwitz G, Nekljudova V, Schmatloch S, Schrenk P, Staebler A, Untch M (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14(7):609–618. https://doi.org/10.1016/s1470-2045(13)70166-9
Acknowledgements
We would like to acknowledge Tonia Parker and Julie Vincuilla for maintaining our institutional database, and Valerie Hope Goldstein for editing this manuscript.
Funding
This work was funded in part by the Dana-Farber/Harvard Cancer Center Breast Specialized Program of Research Excellence (SPORE), an NCI-funded program, Grant No. 1P50CA168504. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health/NCI. EAM receives support from the Rob and Karen Hale Distinguished Chair in Surgical Oncology.
Author information
Authors and Affiliations
Contributions
AW: collected and analyzed the data and wrote the manuscript. CK and SG: collected the data. LP, SR, FN, LD, and EAM: provided critical review of the manuscript. TAK: provided critical oversight during data analysis and provided critical review of the manuscript. All authors approved the final submitted version.
Corresponding author
Ethics declarations
Conflict of interest
AW reports institutional research funding from Myriad Laboratories Inc. EAM: reports clinical trial funding from Roche/Genentech (via SU2C grant) and Gilead; compensated advisory board service for, and consulting fees from, Roche/Genentech, Merck, and Exact Sciences; compensated advisory board service for AstraZeneca; honoraria from Physicians’ Education Resource; and uncompensated service on the Board of Directors for the American Society of Clinical Oncology, the steering committees of Roche/Genentech, Bristol-Myers Squibb, and Eli Lilly, and uncompensated service as a Scientific Advisor for Susan G. Komen for the Cure Foundation. TAK reports Speakers honoraria and an advisory board role for Exact Sciences; Faculty, PrecisCA cancer information service; and is on the global advisory board of Besins Healthcare.
Ethics approval
Institutional Quality Improvement approval was obtained prior to data review and manuscript preparation.
Consent to participate
The need for informed consent was waived for this study; this was reviewed and approved as a quality improvement project by our institution.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Weiss, A., King, C., Vincuilla, J. et al. Rates of pathologic nodal disease among cN0 and cN1 patients undergoing routine axillary ultrasound and neoadjuvant chemotherapy. Breast Cancer Res Treat 195, 181–189 (2022). https://doi.org/10.1007/s10549-022-06677-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06677-2