Abstract
Purpose
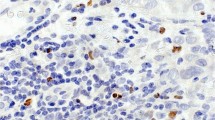
To evaluate the clinical role of tumor-associated macrophages, including foamy (FM) and hemosiderin-laden macrophages (HLM) in the tumor bed (TB) of triple-negative breast cancer (TNBC) post-neoadjuvant chemotherapy (NACT).
Methods
We conducted a pathologic review of 129 women, diagnosed with TNBC between 2002 and 2016 at our institute. The residual cancer burden (RCB) was calculated. We estimated the percentage of tumor-infiltrating lymphocytes (TILs) in the core needle biopsy (CNB), and FM, HLM, and TILs (in TB) [the combined cells are designated as tumor-associated mononuclear cells (TAMNC)]. The information on patient demographics, chemotherapy regimen, recurrence-free survival (RFS), and overall survival (OS) was extracted from the medical records.
Results
Pathologic complete response (pCR) was achieved in 34.1% of the women. TILs (10% increment in CNB) only were associated with pCR in the multivariable analysis [odds ratio 1.04 (1.02, 1.06) (p = 0.0003)]. Immune cells associated with better OS included TAMNC (≤ 30%) [hazard ratio (HR) 4.32 (1.93, 9.66) (p = 0.0004)], and FM (0%) [HR 2.30 (1.06, 4.98) (p = 0.036)]. While increased HLM (10% increment) was statistically significant with HR 0.93 and 95% CI (0.88 to 0.98) (p = 0.0061), using a cutoff of 0%, HLM (0%: negative vs. ≥ 1%: positive) achieved only borderline significance with HR 2.05 (0.98, 4.31) (p = 0.058). Similarly, these immune cells were also associated with better RFS: TAMNC (≤ 30%) [HR 4.57 (2.04, 10.21) (p = 0.0002)], FM (0%) [HR 2.80 (1.23, 6.35) (p = 0.014)], and HLM (0%) [HR 2.34 (1.07, 5.11) (p = 0.03)]. TILs (in TB) were not associated with any clinical outcomes.
Conclusions
Although TILs may play a role in the response to NACT, they may not be critical to the prognosis after NACT. Instead, FM and HLM may assume this role. More studies are warranted.



Similar content being viewed by others
Data availability
Enquiries about data availability should be directed to the authors.
References
Prowell TM, Pazdur R (2012) Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med 366(26):2438–2441
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA et al (2008) Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol 26(8):1275–1281
Dieci MV, Radosevic-Robin N, Fineberg S, van den Eynden G, Ternes N, Penault-Llorca F et al (2018) Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: a report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol 52(Pt 2):16–25
Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C et al (2015) Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol 1(4):448–454
Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE et al (2018) Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19(1):40–50
Pastorello RG, Laws A, Grossmith S, King C, McGrath M, Mittendorf EA et al (2021) Clinico-pathologic predictors of patterns of residual disease following neoadjuvant chemotherapy for breast cancer. Mod Pathol 34(5):875–882
Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schröder CP (2018) Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat Rev 70:178–189
Lissbrant IF, Stattin P, Wikstrom P, Damber JE, Egevad L, Bergh A (2000) Tumor associated macrophages in human prostate cancer: relation to clinicopathological variables and survival. Int J Oncol 17(3):445–496
Kerr KM, Johnson SK, King G, Kennedy MM, Weir J, Jeffrey R (1998) Partial regression in primary carcinoma of the lung: does it occur? Histopathology 33(1):55–63
Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST (1997) Monocyte chemoattractant protein-1 expression and macrophage infiltration in gliomas. Acta Neuropathol 93(5):518–527
Riethdorf L, Riethdorf S, Gützlaff K, Prall F, Löning T (1996) Differential expression of the monocyte chemoattractant protein-1 gene in human papillomavirus-16-infected squamous intraepithelial lesions and squamous cell carcinomas of the cervix uteri. Am J Pathol 149(5):1469–1476
Boström MM, Irjala H, Mirtti T, Taimen P, Kauko T, Ålgars A et al (2015) Tumor-associated macrophages provide significant prognostic information in urothelial bladder cancer. PLoS ONE 10(7):e0133552
Yang M, Li Z, Ren M, Li S, Zhang L, Zhang X et al (2018) Stromal infiltration of tumor-associated macrophages conferring poor prognosis of patients with basal-like breast carcinoma. J Cancer 9(13):2308–2316
Jamiyan T, Kuroda H, Yamaguchi R, Abe A, Hayashi M (2020) CD68- and CD163-positive tumor-associated macrophages in triple negative cancer of the breast. Virchows Arch 477(6):767–775
Medrek C, Pontén F, Jirström K, Leandersson K (2012) The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12:306
Hortobagyi GNCJ, D'Orsi CJ, Edge AB, Mittendorf EA, Rugo LJ, Donald JS, Weaver DL, Winchester DJ, Giuliano A (2017) Breast. In: MB A, (ed). AJCC Cancer Staging Manual. Switzerland: Springer. pp 589–628
Khoury T, Huang X, Chen X, Wang D, Liu S, Opyrchal M (2016) Comprehensive histologic scoring to maximize the predictability of pathology-generated equation of breast cancer oncotype DX recurrence score. Appl Immunohistochem Mol Morphol 24(10):703–711
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G et al (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26(2):259–271
Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V et al (2007) Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25(28):4414–4422
Gönen CSSM (2013) Optimal cutpoint estimation with censored data. J Stat Theory Pract 7(2):345–359
Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A et al (2007) Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13(9):1050–1059
Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN et al (2014) Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol 32(27):2959–2966
Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Van Eenoo F et al (2013) Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol 31(7):860–867
Khoury T, Nagrale V, Opyrchal M, Peng X, Wang D, Yao S (2017) Prognostic significance of stromal versus intratumoral infiltrating lymphocytes in different subtypes of breast cancer treated with cytotoxic neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol
Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M et al (2010) Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol 28(1):105–113
Khoury T, Peng X, Yan L, Wang D, Nagrale V (2018) Tumor-infiltrating lymphocytes in breast cancer: evaluating interobserver variability, heterogeneity, and fidelity of scoring core biopsies. Am J Clin Pathol 150(5):441–450
Luen SJ, Salgado R, Dieci MV, Vingiani A, Curigliano G, Gould RE et al (2019) Prognostic implications of residual disease tumor-infiltrating lymphocytes and residual cancer burden in triple-negative breast cancer patients after neoadjuvant chemotherapy. Ann Oncol 30(2):236–242
Fernandez-Martinez A, Krop IE, Hillman DW, Polley MY, Parker JS, Huebner L et al (2020) Survival, pathologic response, and genomics in CALGB 40601 (Alliance), a neoadjuvant phase III trial of paclitaxel-trastuzumab with or without lapatinib in HER2-positive breast cancer. J Clin Oncol 38(35):4184–4193
Lee H, Lee M, Seo JH, Gong G, Lee HJ (2020) Changes in tumor-infiltrating lymphocytes after neoadjuvant chemotherapy and clinical significance in triple negative breast cancer. Anticancer Res 40(4):1883–1890
Luen SL, Salgado R, Loi S (2019) Residual disease and immune infiltration as a new surrogate endpoint for TNBC post neoadjuvant chemotherapy. Oncotarget 10(45):4612–4614
Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M et al (2017) Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 35(10):1049–1060
Yau C, Osdoit M, van der Noordaa M, Shad S, Wei J, de Croze D et al (2022) Residual cancer burden after neoadjuvant chemotherapy and long-term survival outcomes in breast cancer: a multicentre pooled analysis of 5161 patients. Lancet Oncol 23(1):149–160
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376(22):2147–2159
Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P et al (2021) Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 384(25):2394–2405
Reddy SM, Barcenas CH, Sinha AK, Hsu L, Moulder SL, Tripathy D et al (2018) Long-term survival outcomes of triple-receptor negative breast cancer survivors who are disease free at 5 years and relationship with low hormone receptor positivity. Br J Cancer 118(1):17–23
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821
Funding
The funded was provided by Foundation for the National Institutes of Health, Grant No. (p30ca016056).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Thaer Khoury serves as a pathology faculty advisor for HER2 testing in AstraZeneca. The rest of the authors have nothing to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10549_2022_6641_MOESM1_ESM.pptx
Supplementary Figure 1: Association between post-NACT cells (TILs and TAMNC) percentages and post-NACT variables: A) TILs and pCR status; B) TAMNC and pCR status; D) TILs and RCB class; and D) TAMNC and RCB class. (PPTX 699 kb)
10549_2022_6641_MOESM3_ESM.pptx
Supplement Figure 3: KM curves comparing the outcomes between pCR and the three RCB classes; A) OS and B) RFS (PPTX 251 kb)
10549_2022_6641_MOESM4_ESM.pptx
Supplement Figure 4: KM curve for TAMNC correlating with OS (A) and RFS (B) after excluding cases with pCR (PPTX 167 kb)
Rights and permissions
About this article
Cite this article
Khoury, T., Aljabab, S., Yao, S. et al. Tumor-associated mononuclear cells in the tumor bed of triple-negative breast cancer associate with clinical outcomes in the post-neoadjuvant chemotherapy setting. Breast Cancer Res Treat 194, 531–540 (2022). https://doi.org/10.1007/s10549-022-06641-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06641-0