Abstract
Purpose
Ipsilateral breast tumor recurrence (IBTR) after breast-conserving therapy is seen after a long interval, but the clinical classification of Residual Tumor Recurrence (RR) or Double Primary (DP) needs to be validated. We used genome profiling to identify the genetic alterations associated with IBTR.
Methods
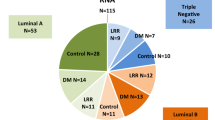
Among 1881 breast cancer patients treated with breast-conserving therapy between 1999 and 2018, IBTR occurred in 52 patients (2.8%). Of these 22 patients who consented for genomic analysis of Primary Breast Cancer (T1) and IBTR (T2) were studied. When the same gene mutations in T1 and T2 were identified, it was classified as genomic residual recurrence gRR, and when no shared mutations identified, it was classified as gDP. The differences between clinical and genomic classification were compared. Furthermore, the pathway of the genes which were responsible for recurrence was also examined.
Results
Of 13 clinically diagnosed RRs (cRRs), 11 were gRR and 2 were gDPs, while of 9 cDPs, 6 were gDP and 3 gRR, with a match rate of 17/22 (77%). We searched for genes involved in IBTR: PIK3CA-AKT pathway mutations were found in 12 of 14 gRRs (86%) in T1, and only 2 of 8 gDPs (25%) with significant difference (p = 0.004). When both of PBC and IBTR compared, PIK3CA-AKT pathway abnormalities were 24/28 (86%) in the gRR and 5/16 (31%) in the gDP (p < 0.001).
Conclusions
Genome profiling revealed that abnormalities in the PIK3CA-AKT pathway in long-term residential recurrences and are a crucial molecular group in the development of IBTR.



Similar content being viewed by others
Data availability
The datasets analyzed during current study are available from the corresponding author on reasonable request.
Abbreviations
- BCT:
-
Breast-conserving therapy
- BCS:
-
Breast-conserving surgery
- PBC:
-
Primary breast cancer
- IBTR:
-
Ipsilateral breast tumor recurrence
References
Veronesi U, Saccozzi R, Del Vecchio M et al (1981) Comparing radical mastectomy with quadrantectomy, axillary dissection, and radiotherapy in patients with small cancers of the breast. N Engl J Med 305:6–11. https://doi.org/10.1056/NEJM198107023050102
Fisher B, Bauer M, Margolese R et al (1985) Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med 312:665–673. https://doi.org/10.1056/NEJM198503143121101
Clarke M, Collins R, Darby S et al (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366:2087–2106. https://doi.org/10.1016/S0140-6736(05)67887-7
Bosma SC, van der Leij F, van Werkhoven E et al (2016) Very low local recurrence rates after breast-conserving therapy: analysis of 8485 patients treated over a 28-year period. Breast Cancer Res Treat 156:391–400. https://doi.org/10.1007/s10549-016-3732-0
Ishitobi M, Komoike Y, Motomura K, Koyama H, Inaji H (2010) Early response to neo-adjuvant chemotherapy in carcinoma of the breast predicts both successful breast-conserving surgery and decreased risk of ipsilateral breast tumor recurrence. Breast J 16:9–13. https://doi.org/10.1111/j.1524-4741.2009.00864.x
van Laar C, van der Sangen MJ, Poortmans PM et al (2013) Local recurrence following breast-conserving treatment in women aged 40 years or younger: trends in risk and the impact on prognosis in a population-based cohort of 1143 patients. Eur J Cancer 49:3093–3101. https://doi.org/10.1016/j.ejca.2013.05.030
National Comprehensive Cancer Network. available at https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf accessed 29,Jan. 2022.
Guidelines of Japanese Breast Cancer society. available at https://jbcs.gr.jp/guidline/2018/index/ accessed 29, Jan, 2022.
Krauss DJ, Kestin LL, Mitchell C, Martinez AA, Vicini FA (2004) Changes in temporal patterns of local failure after breast-conserving therapy and their prognostic implications. Int J Radiat Oncol Biol Phys 60:731–740. https://doi.org/10.1016/j.ijrobp.2004.04.010
Huang E, Buchholz TA, Meric F et al (2002) Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer 95:2059–2067. https://doi.org/10.1002/cncr.10952
Goto T, Hirotsu Y, Mochizuki H et al (2017) Mutational analysis of multiple lung cancers: Discrimination between primary and metastatic lung cancers by genomic profile. Oncotarget 8:31133–31143. https://doi.org/10.18632/oncotarget.16096
Hirotsu Y, Hada M, Amemiya K, Oyama T, Mochizuki H, Omata M (2020) Multi-regional sequencing reveals clonal and polyclonal seeding from primary tumor to metastases in advanced gastric cancer. J Gastroenterol 55:553–564. https://doi.org/10.1007/s00535-019-01659-6
Takano A, Hirotsu Y, Amemiya K et al (2017) Genetic basis of a common tumor origin in the development of pancreatic mixed acinar-neuroendocrine-ductal carcinoma: A case report. Oncol Lett 14:4428–4432. https://doi.org/10.3892/ol.2017.6786
Higuchi R, Nakagomi T, Goto T et al (2020) Identification of clonality through Genomic Profile Analysis In Multiple Lung Cancers. J Clin Med. https://doi.org/10.3390/jcm9020573
Goto T, Hirotsu Y, Oyama T, Amemiya K, Omata M (2016) Analysis of tumor-derived DNA in plasma and bone marrow fluid in lung cancer patients. Med Oncol 33:29
Hirotsu Y, Zheng TH, Amemiya K, Mochizuki H, Guleng B, Omata M (2016) Targeted and exome sequencing identified somatic mutations in hepatocellular carcinoma. Hepatol Res 5:12663
Amemiya K, Hirotsu Y, Goto T et al (2016) Touch imprint cytology with massively parallel sequencing (TIC-seq): a simple and rapid method to snapshot genetic alterations in tumors. Cancer Med 5:3426–3436. https://doi.org/10.1002/cam4.950
Rodenhiser DI et al (2012) Comprehensive molecular portraits of human breast tumours. Nature 490:61–70. https://doi.org/10.1038/nature11412
Forbes SA, Beare D, Gunasekaran P et al (2015) COSMIC: exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res 43:D805-811. https://doi.org/10.1093/nar/gku1075
Toy W, Shen Y, Won H et al (2013) ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nat Genet 45:1439–1445. https://doi.org/10.1038/ng.2822
Hirotsu Y, Nakagomi H, Sakamoto I et al (2015) Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Mol Genet Genomic Med 3:459–466
Vicini FA, Antonucci JV, Goldstein N et al (2007) The use of molecular assays to establish definitively the clonality of ipsilateral breast tumor recurrences and patterns of in-breast failure in patients with early-stage breast cancer treated with breast-conserving therapy. Cancer 109:1264–1272. https://doi.org/10.1002/cncr.22529
Sun GY, Wang SL, Tang Y et al (2018) Clinical characteristics and prognosis of patients with ipsilateral breast tumor recurrence after breast conservation therapy. Zhonghua Zhong Liu Za Zhi 40:352–358. https://doi.org/10.3760/cma.j.issn.0253-3766.2018.05.007
Bollet MA, Servant N, Neuvial P et al (2008) High-resolution mapping of DNA breakpoints to define true recurrences among ipsilateral breast cancers. J Natl Cancer Inst 100:48–58. https://doi.org/10.1093/jnci/djm266
Cancer Genome Atlas N (2012) Comprehensive molecular portraits of human breast tumours. Nature 490:61–70. https://doi.org/10.1038/nature11412
Li H, Prever L, Hirsch E, Gulluni F (2021) Targeting PI3K/AKT/mTOR Signaling Pathway in Breast Cancer. Cancers (Basel). https://doi.org/10.3390/cancers13143517
Mosele F, Stefanovska B, Lusque A et al (2020) Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann Oncol 31:377–386. https://doi.org/10.1016/j.annonc.2019.11.006
Van Keymeulen A, Lee MY, Ousset M et al (2015) Reactivation of multipotency by oncogenic PIK3CA induces breast tumour heterogeneity. Nature 525:119–123. https://doi.org/10.1038/nature14665
Koren S, Reavie L, Couto JP et al (2015) PIK3CA(H1047R) induces multipotency and multi-lineage mammary tumours. Nature 525:114–118. https://doi.org/10.1038/nature14669
Lin CY, Vennam S, Purington N et al (2019) Genomic landscape of ductal carcinoma in situ and association with progression. Breast Cancer Res Treat 178:307–316. https://doi.org/10.1007/s10549-019-05401-x
Gener P, Rafael D, Seras-Franzoso J et al (2019) Pivotal role of AKT2 during dynamic phenotypic change of breast cancer stem cells. Cancers (Basel). https://doi.org/10.3390/cancers11081058
Ginter PS, D’Alfonso TM (2017) Current concepts in diagnosis, molecular features, and management of lobular carcinoma in situ of the breast with a discussion of morphologic variants. Arch Pathol Lab Med 141:1668–1678. https://doi.org/10.5858/arpa.2016-0421-RA
Ciriello G, Gatza ML, Beck AH et al (2015) Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 163:506–519. https://doi.org/10.1016/j.cell.2015.09.033
Vallette FM, Olivier C, Lezot F et al (2019) Dormant, quiescent, tolerant and persister cells: Four synonyms for the same target in cancer. Biochem Pharmacol 162:169–176. https://doi.org/10.1016/j.bcp.2018.11.004
Acknowledgements
Not applicable.
Funding
This study was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (B) (Grant Number 20H03668 to YH), Early-Career Scientists (Grant Number JP18K16292 to YH), and Research Grant for Young Scholars (to YH), the YASUDA Medical Foundation (to YH), the Uehara Memorial Foundation (to YH), the Medical Research Grants from the Takeda Science Foundation (to YH), and a Grant-in-Aid for Genome Research Project from Yamanashi Prefecture (to MO and YH).
Author information
Authors and Affiliations
Contributions
All Authors contributed to the study conception and design. Material collection and data collection were performed by HN, MI and analysis were performed by YH, KA and HM. The first draft of the manuscript was written by HN and MO, and all authors commented on previous version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Ethical approval
This study was approved by the institutional review board in our hospital (No. 2018–6). We analyzed 22 cases in whom written informed consent for genomic analysis was obtained.
Consent for publication
Consent for publication was included in the written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakagomi, H., Inoue, M., Hirotsu, Y. et al. PIK3CA-AKT pathway predominantly acts in developing ipsilateral breast tumor recurrence long after breast-conserving surgery. Breast Cancer Res Treat 193, 349–359 (2022). https://doi.org/10.1007/s10549-022-06570-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-022-06570-y