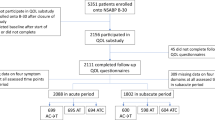
Abstract
Background
The NSABP B-36 compared four cycles of doxorubicin and cyclophosphamide (AC) with six cycles of 5-fluorouracil, epirubicin, and cyclophosphamide (FEC-100) in node-negative early-stage breast cancer. A sub-study within B-36, focusing on symptoms, quality of life (QOL), menstrual history (MH), and cardiac function (CF) was conducted.
Patients and methods
Patients completed the QOL questionnaire at baseline, during treatment, and every 6 months through 36 months. FACT-B Trial Outcome Index (TOI), symptom severity, and SF-36 Vitality and Physical Functioning (PF) scales scores were compared between the two groups using a mixed model for repeated measures analysis. MH was collected at baseline and subsequently assessed if menstrual bleeding occurred within 12 months prior to randomization. Post-chemotherapy amenorrhea outcome was examined at 18 months and was defined as lack of menses in the preceding year. Logistic regression was used to test for association of amenorrhea and treatment. CF assessment was done at baseline and 12 months. Correlation analysis was used to address associations between changes in baseline and 12-month PF and concurrent CF changes measured by LVEF.
Results
FEC-100 patients had statistically significantly lower TOI scores during chemotherapy (P = 0.02) and at 6 months (P < 0.001); lower Vitality score at 6 months (P < 0.01), and lower PF score during the first year than AC patients. There were no statistically significant QOL score differences between the two groups beyond 12 months. No significant differences in symptom severity between the two groups were observed. Rates of amenorrhea were significantly different between FEC-100 and AC (67.4% vs. 59.1%, P < 0.001). There was no association between changes in LVEF and PF (P = 0.38).
Conclusions
Statistically significant QOL differences between the two groups favored AC; however, the magnitude was small and unlikely to be clinically meaningful. There was a clinical and statistically significant difference in risk for amenorrhea, favoring AC.
Trial registry
NCT00087178; Date of registration: 07/08/2004.




Similar content being viewed by others
Data availability
Individual participant data that underlie the results reported in this article, after deidentification, will generally be available within 1 year after publication and will be accessible through the NCTN Data Archive.
Code availability
Analyses were performed using SAS standard procedures (v9.4; SAS Institute, Cary, NC.
References
Paik S, Shak S, Tang, et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826. https://www.ncbi.nlm.nih.gov/pubmed/?term=15591335
Paik S, Tang G, Shak S, et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24:3726–3734. https://www.ncbi.nlm.nih.gov/pubmed/?term=16720680
Early stage breast cancer. Consens Statement 8(6):1–19. Review. https://www.ncbi.nlm.nih.gov/pubmed/2247093. Accessed 18–21 Jun 1990
Brady MJ, Cella DF, Mo F, et al (1997) Reliability and validity of the functional assessment of cancer therapy-breast quality-of-life instrument. J Clin Oncol 15:974–986. https://www.ncbi.nlm.nih.gov/pubmed/?term=9060536
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483. https://www.ncbi.nlm.nih.gov/pubmed/?term=1593914
Ganz PA, Land SR, Geyer CE Jr, et al (2011) Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol 29:1110–1116. https://www.ncbi.nlm.nih.gov/pubmed/?term=PMC3083866
Land SR, Kopec JA, Yothers G, Anderson S, et al (2004) Health-related quality of life in axillary node-negative, estrogen receptor-negative breast cancer patients undergoing AC versus CMF chemotherapy: findings from the national surgical adjuvant breast and bowel project B-23. Breast Cancer Res Treat 86:153–164. https://www.ncbi.nlm.nih.gov/pubmed/?term=15319567
Eton DT, Cella D, Yost KJ, et al (2004) A combination of distribution- and anchor-based approaches determined minimally important differences (MIDs) for four endpoints in a breast cancer scale. J Clin Epidemiol 57:898–910. https://www.ncbi.nlm.nih.gov/pubmed/?term=15504633
Ganz PA, Day R, Ware JE Jr, Redmond C, Fisher B (1995) Base-line quality-of-life assessment in the national surgical adjuvant breast and bowel project breast cancer prevention trial. J Natl Cancer Inst 87(18):1372–1382. https://www.ncbi.nlm.nih.gov/pubmed/?term=7658498
Cella D, Land SR, Chang CH, et al (2008) Symptom measurement in the breast cancer prevention trial (BCPT) (P-1): psychometric properties of a new measure of symptoms for midlife women. Breast Cancer Res Treat 109:515–526. https://www.ncbi.nlm.nih.gov/pubmed/?term=17851765
Stanton AL, Bernaards CA, Ganz PA (2005) The BCPT symptom scales: a measure of physical symptoms for women diagnosed with or at risk for breast cancer. J Natl Cancer Inst 97:448–456. https://www.ncbi.nlm.nih.gov/pubmed/?term=15770009
Land SR, Ritter MW, Costantino JP, et al (2007) Compliance with patient-reported outcomes in multicenter clinical trials: methodologic and practical approaches. J Clin Oncol 25:5113–5120. https://www.ncbi.nlm.nih.gov/pubmed/?term=17991930
Geyer, Jr. CE, Bandos H, Rastogi P, et al Definitive results of a phase III adjuvant trial comparing six cycles of FEC-100 to four cycles of AC in women with operable node-negative breast cancer: the NSABP B-36 Trial (NRG Oncology). https://doi.org/10.1007/s10549-021-06417-y
Ganz PA, Cecchini RS, Julian TB, et al (2016) Patient-reported outcomes with anastrozole versus tamoxifen for postmenopausal patients with ductal carcinoma in situ treated with lumpectomy plus radiotherapy (NSABP B-35): a randomised, double-blind, phase 3 clinical trial. Lancet 387(10021):857–865. https://pubmed.ncbi.nlm.nih.gov/26686960/
Silva C, Caramelo O, Almeida-Santos T, Ribeiro Rama AC (2016) Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Hum Reprod 31:2737–2749. https://www.ncbi.nlm.nih.gov/pubmed/?term=27664208
Acknowledgements
The authors would also like to acknowledge John Wilson, PhD (retired), who was the original Protocol Statistician for the study and was an invaluable part of the protocol team. The authors acknowledge the contributions of Barbara C. Good, PhD, Director of Scientific Publications, Christine I. Rudock, Publications and Graphics Specialist, and Wendy L. Rea, BA, Editorial Associate, all of whom are employees of NSABP. They were not compensated beyond their normal salaries for this work.
Funding
This work was supported by the National Institutes of Health grants U10CA180868, UG1CA189867, -180822, and -44066-26 (Dr. Robidoux), and Pharmacia & Upjohn Company, a subsidiary of Pfizer, Inc. The funders had no role in the design of the study; the collection, analysis, and/or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: PAG, HB, CEG, LF, JTH, RGM, and NW. Methodology: PAG, HB, CEG, and RGM. Software: HB. Validation: PAG, HB, and EPM. Formal Analysis: HB. Investigation: PAG, CEG, AR, JP, LB-D, AMB, LF, AWP, PJW, LP, JTH, PJS, RGM, HRS, EPM, and NW. Resources: AR, AHGP, JP, AMB, LF, AWP, and LP. Data curation: HB and AR. Writing—original draft: PAG, HB, CEG, and AMB. Writing—review & editing: PAG, HB, CEG, AHGP, JP, AMB, LF, PJW, LP, JTH, PJS, RLC, RGM, HRS, EPM, and NW. Visualization: HB, CEG, RGM, and EPM. Supervision: CEG, LF, LP, EPM, and NW. Project administration: CEG, AWP, and NW. Funding acquisition: NW.
Corresponding author
Ethics declarations
Conflict of interest
Charles E. Geyer, Jr.—Grants, non-financial support and other from Genentech/Roche, Daiichi/Sankyo, and AstraZeneca, during the conduct of the study, and personal fees from Exact Sciences and Athenex, outside the submitted work. Johnathan Polikoff—Consultant, Natera. Louis Provencher—Consulting or Advisory Role: Lilly, Pfizer, Roche, and Novartis. Research Funding: Pfizer, Roche, Novartis, Merck, GlaxoSmithKline, and Odonate Therapeutics. The remaining authors declare no conflicts of interest.
Ethical approval
The protocol was approved by institutional IRBs. All studies involving human participants are in accord with ethical standards of the institutional research board and the Helsinki declaration or comparable ethical standards.
Consent to participate
The study was approved by the local institutional review board. Written informed consent was required of all participants.
Consent for publication
All authors have consented for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ganz, P.A., Bandos, H., Geyer, C.E. et al. Behavioral and health outcomes from the NRG Oncology/NSABP B-36 trial comparing two different adjuvant therapy regimens for early-stage node-negative breast cancer. Breast Cancer Res Treat 192, 153–161 (2022). https://doi.org/10.1007/s10549-021-06475-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06475-2