Abstract
Purpose
In clinically node-positive breast cancer, axillary staging after neoadjuvant chemotherapy (NAC) is optimized with targeted axillary dissection (TAD), which includes removal of the biopsy-proven metastatic lymph node (LN) in addition to sentinel lymph nodes (SLN). Localization of the clipped node is currently performed post-NAC; however, technical limitations can make detection and localization of the treated LN challenging. We prospectively evaluated the feasibility of localizing the metastatic LN with a SAVI SCOUT® reflector (SAVI) prior to NAC for targeted removal at surgery.
Methods
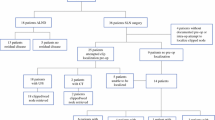
Twenty-five patients with stage 2/3 breast cancer underwent ultrasound-guided localization of the biopsy-proven LN with SAVI prior to NAC. After NAC, patients with clinical response underwent TAD. Primary outcome measures were rate of successful localization, days between insertion of SAVI and axillary surgery, frequency of retrieval of clipped node, and frequency of SAVI-LN as SLN.
Results
After NAC, 23/25 (92%) had clinical axillary down-staging and underwent TAD. Two patients with persistent palpable axillary disease underwent ALND for initial staging. Axillary surgery was performed at an average of 141 days post-SAVI insertion and the SAVI was successfully retrieved in all cases. Among 23 patients undergoing TAD, the SAVI was retrieved within a LN in all patients, whereas clip migration was observed in two patients. The median SLN removed was 4, and SAVI-LN was SLN in 22/23 patients. Axillary pCR rate was 44%.
Conclusion
Localizing a metastatic LN with SAVI reflector prior to NAC for targeted removal at surgery is feasible and may provide technical and logistical advantages over axillary localization post-NAC.
Clinical trial registry
Clinical trials.gov identifier: NCT03411070


Similar content being viewed by others
References
Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC (2018) Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2+ subtypes. Ann Surg Oncol 25(8):2241–2248
Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Flippo-Morton T, Hunt KK (2014) Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg 260(4):608–614 (Discussion 614–606)
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, Lebeau A, Liedtke C, von Minckwitz G, Nekljudova V et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14(7):609–618
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Leitch AM, Kuerer HM, Bowling M, Flippo-Morton TS et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 310(14):1455–1461
Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, Meterissian S, Arnaout A, Brackstone M, McCready DR et al (2015) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 33(3):258–264
Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, Feliberti EC, Hunt KK (2016) Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0–T4, N1–N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg 263(4):802–807
National comprehensive cancer network. Breast cancer, version 3.2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 10 July 2021
Caudle AS, Yang WT, Mittendorf EA, Black DM, Hwang R, Hobbs B, Hunt KK, Krishnamurthy S, Kuerer HM (2015) Selective surgical localization of axillary lymph nodes containing metastases in patients with breast cancer: a prospective feasibility trial. JAMA Surg 150(2):137–143
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, Bedrosian I, Hobbs BP, DeSnyder SM, Hwang RF et al (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34(10):1072–1078
Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC (2017) Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and challenges. Ann Surg Oncol 24(10):3011–3016
Laws A, Dillon K, Kelly BN, Kantor O, Hughes KS, Gadd MA, Smith BL, Lamb LR, Specht M (2020) Node-positive patients treated with neoadjuvant chemotherapy can be spared axillary lymph node dissection with wireless non-radioactive localizers. Ann Surg Oncol 27(12):4819–4827
Man V, Kwong A (2021) Different strategies in marking axillary lymph nodes in breast cancer patients undergoing neoadjuvant medical treatment: a systematic review. Breast Cancer Res Treat 186(3):607–615
Plecha D, Bai S, Patterson H, Thompson C, Shenk R (2015) Improving the accuracy of axillary lymph node surgery in breast cancer with ultrasound-guided wire localization of biopsy proven metastatic lymph nodes. Ann Surg Oncol 22(13):4241–4246
Donker M, Straver ME, Wesseling J, Loo CE, Schot M, Drukker CA, van Tinteren H, Sonke GS, Rutgers EJ, Vrancken Peeters MJ (2015) Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg 261(2):378–382
Lee MK, Sanaiha Y, Kusske AM, Thompson CK, Attai DJ, Baker JL, Fischer CP, DiNome ML (2020) A comparison of two non-radioactive alternatives to wire for the localization of non-palpable breast cancers. Breast Cancer Res Treat 182(2):299–303
Mariscal Martinez A, Vives Rosello I, Salazar Gomez A, Catanese A, Perez Molina M, Sola Suarez M, Pascual Miguel I, Blay Aulina L, Rios Gozalvez C, Julian Ibanez JF et al (2021) Advantages of preoperative localization and surgical resection of metastatic axillary lymph nodes using magnetic seeds after neoadjuvant chemotherapy in breast cancer. Surg Oncol 36:28–33
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172
Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, Kuroi K, Im SA, Park BW, Kim SB et al (2017) Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376(22):2147–2159
Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE Jr, Taghian A, Wickerham DL, Wolmark N (2012) Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J Clin Oncol 30(32):3960–3966
Wong SM, Almana N, Choi J, Hu J, Gagnon H, Natsuhara K, Shen AH, DeSantis S, Dominici L, Golshan M et al (2019) Prognostic significance of residual axillary nodal micrometastases and isolated tumor cells after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol 26(11):3502–3509
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, Wolmark N, Rastogi P, Schneeweiss A, Redondo A et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380(7):617–628
Sun J, Henry DA, Carr MJ, Yazdankhahkenary A, Laronga C, Lee MC, Hoover SJ, Sun W, Czerniecki BJ, Khakpour N et al (2021) Feasibility of axillary lymph node localization and excision using radar reflector localization. Clin Breast Cancer. https://doi.org/10.1016/j.clbc.2020.08.001
Diego EJ, McAuliffe PF, Soran A, McGuire KP, Johnson RR, Bonaventura M, Ahrendt GM (2016) Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol 23(5):1549–1553
United States Nuclear Regulatory Commission (2017) Iodine-125 and palladium-103 low dose rate brachytherapy seeds used for localization of non-palpable lesions. https://www.nrc.gov/materials/miau/med-use-toolkit/seed-localization.html. Accessed 10 July 2021
Garcia-Moreno JL, Benjumeda-Gonzalez AM, Amerigo-Gongora M, Landra-Dulanto PJ, Gonzalez-Corena Y, Gomez-Menchero J (2019) Targeted axillary dissection in breast cancer by marking lymph node metastasis with a magnetic seed before starting neoadjuvant treatment. J Surg Case Rep 11:rjz344
Pilewskie M, Morrow M (2017) Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol 3(4):549–555
Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M, El-Tamer M, Gemignani ML, Heerdt AS, Sclafani LM et al (2016) How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol 23(11):3467–3474
Choy N, Lipson J, Porter C, Ozawa M, Kieryn A, Pal S, Long Trinh J, Wheeler A, Ikeda D, Jensen K et al (2014) Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer patients. Ann Surg Oncol 22(2):377–382
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baker, J.L., Haji, F., Kusske, A.M. et al. SAVI SCOUT® localization of metastatic axillary lymph node prior to neoadjuvant chemotherapy for targeted axillary dissection: a pilot study. Breast Cancer Res Treat 191, 107–114 (2022). https://doi.org/10.1007/s10549-021-06416-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06416-z