Abstract
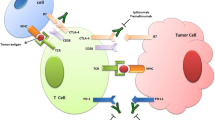
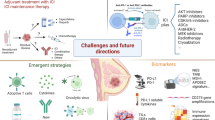
While checkpoint inhibitors have been approved in patients with newly metastatic PDL1-positive triple negative breast cancer, similar clinical benefit with immunotherapy alone or in combination with chemotherapy has not been observed in patients with hormone receptor-positive, HER2− negative breast cancer in the metastatic setting. However, in the ISPY2 trial, an increase in pathologic response has been observed with the addition of immunotherapy (± PARP inhibition) to chemotherapy compared to chemotherapy alone in patients with high-risk hormone receptor-positive, HER2− breast cancer. We review strategies to enhance the immunotherapeutic activity in this subtype of breast cancer, including combinations of checkpoint inhibition with chemotherapy, endocrine therapy, PARP inhibitors, HDAC inhibitors, CDK4/6 inhibitors, and radiotherapy. Combinations with agents targeting novel immunotherapeutic targets are also discussed. Though there remains an unmet need for immunotherapy approaches in patients with hormone-receptor positive breast cancer, there are a number of approaches that may lead to increased anti-tumor activity with immunotherapy in this tumor subtype.

Similar content being viewed by others
References
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G et al (2018) Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379(22):2108–2121
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im SA, Yusof MM, Gallardo C, Lipatov O, Barrios CH, Holgado E et al (2020) Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396(10265):1817–1828
Stanton SE, Adams S, Disis ML (2016) Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: a systematic review. JAMA Oncol 2(10):1354–1360
Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, Zhang Q (2017) Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget 8(19):31347–31354
Rugo HS, Delord J-P, Im S-A, Ott PA, Piha-Paul SA, Bedard PL, Sachdev J, Tourneau CL, van Brummelen EMJ, Varga A et al (2018) Safety and antitumor activity of pembrolizumab in patients with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer. Clin Cancer Res 24(12):2804–2811
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, Boccia R, Lippman ME, Somer R, Smakal M et al (2018) Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat 167(3):671–686
Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin JA, Miller WH Jr et al (2020) Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol 21(10):1353–1365
Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, Lin N, Tolaney SM, Wagle N (2020) Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol 31(3):387–394
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R et al (2019) Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381(16):1535–1546
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora JE et al (2019) Nivolumab plus Ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 381(21):2020–2031
Barroso-Sousa R, Trippa L, Lange P, Andrews C, McArthur HL, Haley BB, Rugo HS, Emens LA, Winer EP, Mittendorf EA et al (2019) Nimbus: a phase II study of nivolumab plus ipilimumab in metastatic hypermutated HER2-negative breast cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS1115
Santa-Maria CA, Kato T, Park JH, Kiyotani K, Rademaker A, Shah AN, Gross L, Blanco LZ, Jain S, Flaum L et al (2018) A pilot study of durvalumab and tremelimumab and immunogenomic dynamics in metastatic breast cancer. Oncotarget 9(27):18985–18996
Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc F, Coussy F, Sablin M-P, Debled M, Lefeuvre-Plesse C et al (2021) Durvalumab compared to maintenance chemotherapy in metastatic breast cancer: the randomized phase II SAFIR02-BREAST IMMUNO trial. Nat Med 27(2):250–255
Krysko O, Love Aaes T, Bachert C, Vandenabeele P, Krysko DV (2013) Many faces of DAMPs in cancer therapy. Cell Death Dis 4:e631
Emens LA, Middleton G (2015) The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res 3(5):436–443
Murciano-Goroff YR, Warner AB, Wolchok JD (2020) The future of cancer immunotherapy: microenvironment-targeting combinations. Cell Res 30(6):507–519
Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF et al (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378(22):2078–2092
Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A et al (2018) Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379(21):2040–2051
Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, Huemer F, Losonczy G, Johnson ML, Nishio M et al (2018) First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 379(23):2220–2229
Burtness B, Harrington KJ, Greil R, Soulieres D, Tahara M, de Castro G, Jr., Psyrri A, Baste N, Neupane P, Bratland A, et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928
Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D (2015) The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 41(6):503–510
Liu Y, Dong Y, Kong L, Shi F, Zhu H, Yu J (2018) Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J Hematol Oncol 11(1):104
Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M et al (2017) Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377(20):1919–1929
Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, Mendez G, Feliciano J, Motoyama S, Lievre A et al (2021) Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N Engl J Med 384(13):1191–1203
Shah AN, Flaum L, Helenowski I, Santa-Maria CA, Jain S, Rademaker A, Nelson V, Tsarwhas D, Cristofanilli M, Gradishar W (2020) Phase II study of pembrolizumab and capecitabine for triple negative and hormone receptor-positive, HER2-negative endocrine-refractory metastatic breast cancer. J Immunother Cancer. https://doi.org/10.1136/jitc-2019-000173
Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, Wulf G, Spring L, Sinclair NF, Andrews C et al (2020) Effect of eribulin with or without pembrolizumab on progression-free survival for patients with hormone receptor-positive, ERBB2-negative metastatic breast cancer: a randomized clinical trial. JAMA Oncol 6(10):1598–1605
Vonderheide RH, LoRusso PM, Khalil M, Gartner EM, Khaira D, Soulieres D, Dorazio P, Trosko JA, Rüter J, Mariani GL et al (2010) Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin Cancer Res 16(13):3485–3494
Hakem R (2008) DNA-damage repair; the good, the bad, and the ugly. EMBO J 27(4):589–605
Ratta R, Guida A, Scotte F, Neuzillet Y, Teillet AB, Lebret T, Beuzeboc P (2020) PARP inhibitors as a new therapeutic option in metastatic prostate cancer: a systematic review. Prostate Cancer Prostatic Dis 23(4):549–560
Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M et al (2018) Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med 379(8):753–763
Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A et al (2017) Olaparib for metastatic breast cancer in patients with a germline brca mutation. N Engl J Med 377(6):523–533
Tung NM, Robson ME, Ventz S, Santa-Maria CA, Nanda R, Marcom PK, Shah PD, Ballinger TJ, Yang ES, Vinayak S et al (2020) TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J Clin Oncol 38(36):4274–4282
Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S et al (2017) DNA double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun 8(1):1751
Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, Hsu JM, Hsu JL, Yu WH, Du Y, Lee HH et al (2017) PARP Inhibitor Upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 23(14):3711–3720
Ding L, Kim HJ, Wang Q, Kearns M, Jiang T, Ohlson CE, Li BB, Xie S, Liu JF, Stover EH et al (2018) PARP inhibition elicits STING-dependent antitumor immunity in Brca1-deficient ovarian cancer. Cell Rep. https://doi.org/10.1016/j.celrep.2018.11.054
Pantelidou C, Sonzogni O, De Oliveria TM, Mehta AK, Kothari A, Wang D, Visal T, Li MK, Pinto J, Castrillon JA et al (2019) PARP inhibitor efficacy depends on CD8(+) T-cell recruitment via intratumoral STING pathway activation in BRCA-deficient models of triple-negative breast cancer. Cancer Discov 9(6):722–737
Shen J, Zhao W, Ju Z, Wang L, Peng Y, Labrie M, Yap TA, Mills GB, Peng G (2019) PARPi triggers the STING-dependent immune response and enhances the therapeutic efficacy of immune checkpoint blockade independent of BRCAness. Cancer Res 79(2):311–319
Domchek SM, Postel-Vinay S, Im SA, Park YH, Delord JP, Italiano A, Alexandre J, You B, Bastian S, Krebs MG et al (2020) Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study. Lancet Oncol 21(9):1155–1164
Yap TA, Konstantinopoulos P, Telli ML, Saraykar S, Beck JT, Galsky MD, Abraham J, Wise DR, Khasraw M, Rubovszky G et al (2020) Abstract P1–19–03: JAVELIN PARP medley, a phase 1b/2 study of avelumab plus talazoparib: results from advanced breast cancer cohorts. Cancer Res. https://doi.org/10.1158/1538-7445.SABCS19-P1-19-03
Finn RS, Aleshin A, Slamon DJ (2016) Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 18(1):17
Finn RS, Martin M, Rugo HS, Jones S, Im S-A, Gelmon K, Harbeck N, Lipatov ON, Walshe JM, Moulder S et al (2016) Palbociclib and letrozole in advanced breast cancer. N Engl J Med 375(20):1925–1936
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im S-A, Petrakova K, Bianchi GV, Esteva FJ, Martín M et al (2019) Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 382(6):514–524
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, Park IH, Trédan O, Chen SC, Manso L et al (2017) MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 35(32):3638–3646
Goel S, DeCristo MJ, Watt AC, BrinJones H, Sceneay J, Li BB, Khan N, Ubellacker JM, Xie S, Metzger-Filho O et al (2017) CDK4/6 inhibition triggers anti-tumour immunity. Nature 548(7668):471–475
Deng J, Wang ES, Jenkins RW, Li S, Dries R, Yates K, Chhabra S, Huang W, Liu H, Aref AR et al (2018) CDK4/6 inhibition augments antitumor immunity by enhancing t-cell activation. Cancer Discov 8(2):216–233
Hurvitz SA, Martin M, Press MF, Chan D, Fernandez-Abad M, Petru E, Rostorfer R, Guarneri V, Huang CS, Barriga S et al (2020) Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR(+)/HER2(-) breast cancer. Clin Cancer Res 26(3):566–580
Rugo HS, Kabos P, Beck JT, Chisamore MJ, Hossain A, Chen Y, Tolaney SM (2020) A phase Ib study of abemaciclib in combination with pembrolizumab for patients with hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) locally advanced or metastatic breast cancer (MBC) (NCT02779751): interim results. J Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl
Masuda J, Tsurutani J, Masuda N, Futamura M, Matsumoto K, Aogi K, Takahashi M, Iwata H, Iwasa T, Mukohara et al (2020) Phase II study of nivolumab in combination with abemaciclib plus endocrine therapy in patients with hormone receptor-positive human epidermal growth factor receptor-2 negative metastatic breast cancer (WJOG11418B, NEWFLAME trial). Abstract PS12-10. In: Virtual San Antonio Breast Cancer Symposium, Texas, 8-11 December 2020.
Bates SE (2020) Epigenetic therapies for cancer. N Engl J Med 383(7):650–663
Yi X, Wei W, Wang SY, Du ZY, Xu YJ, Yu XD (2008) Histone deacetylase inhibitor SAHA induces ERalpha degradation in breast cancer MCF-7 cells by CHIP-mediated ubiquitin pathway and inhibits survival signaling. Biochem Pharmacol 75(9):1697–1705
Terranova-Barberio M, Pawlowska N, Dhawan M, Moasser M, Chien AJ, Melisko ME, Rugo H, Rahimi R, Deal T, Daud A et al (2020) Exhausted T cell signature predicts immunotherapy response in ER-positive breast cancer. Nat Commun 11(1):3584
Barroso-Sousa R, Krop IE, Trippa L, Tan-Wasielewski Z, Li T, Osmani W, Andrews C, Dillon D, Richardson ET 3rd, Pastorello RG et al (2020) A phase II study of pembrolizumab in combination with palliative radiotherapy for hormone receptor-positive metastatic breast cancer. Clin Breast Cancer 20(3):238–245
Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, Bousetta N, Medioni J, Gligorov J, Grygar C et al (2010) First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med 8:71
Wildiers H, Armstrong A, Cuypere E, Dalenc F, Dirix LY, Chan S (2020) Abstract PD14–08: Primary efficacy results from AIPAC: A double-blinded, placebo controlled, randomized multinational phase IIb trial comparing weekly paclitaxel plus eftilagimod alpha (soluble LAG-3 protein) vs. weekly paclitaxel plus placebo in HR-positive metastatic breast cancer patients. In: Virtual San Antonio Breast Cancer Symposium
Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz AB Jr, Fisher ER, Wickerham DL, Wolmark N et al (1997) Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15(7):2483–2493
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. The Lancet 384(9938):164–172
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H et al (2020) Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol 6(5):676–684
Cardoso F, Bardia A, Andre F, Cescon DW, McArthur HL, Telli ML, Loi S, Cortes J, Schmid P, Harbeck N et al (2019) KEYNOTE-756: randomized, double-blind, phase 3 study of pembrolizumab vs placebo combined with neoadjuvant chemotherapy and adjuvant endocrine therapy for high-risk, early-stage estrogen receptor–positive, human epidermal growth factor receptor 2–negative (ER+/HER2−) breast cancer. J Clin Oncol. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS601
Loi S, McArthur HL, Harbeck N, Pusztai L, Delaloge S, Letrent K, Chen T, Li B, Tatsuoka K, Zardavas D et al (2020) A phase III trial of nivolumab with neoadjuvant chemotherapy and adjuvant endocrine therapy in ER+/HER2- primary breast cancer: checkmate 7FL. J Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS604
Pusztai LHH et al (2020) Abstract No. CT011: evaluation of durvalumab in combination with olaparib and paclitaxel in high-risk HER2- stage II/III breast cancer: results from the I-SPY 2 TRIAL. AACR. https://doi.org/10.1158/1538-7445.AM2020-CT011
Tolaney SM, Jerusalem G, Salgado R, Liu X, Chen T, Zhang H, Roberts M, Zardavas D, Prat A (2020) A phase II trial of nivolumab (NIVO) + palbociclib (PAL) + anastrozole (ANA) in postmenopausal women and men with estrogen receptor (ER)+/human epidermal growth factor 2 (HER2)- primary breast cancer (BC): checkmate 7A8. J Clin Oncol. https://doi.org/10.1200/JCO.2020.38.15_suppl.TPS1105
Author information
Authors and Affiliations
Contributions
All authors (MK, JEM, KK) contributed equally to this manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MK and JEM have no disclosures to report. KK declares the following potential conflicts of interest: Medical Advisor—Immunomedics, Pfizer, Novartis, Eisai, Eli-Lilly, Amgen, Immunomedics, Merck, Seattle Genetics, and Astra Zeneca; Institutional Support—Immunomedics, Novartis, Incyte, Genentech/Roche, Eli-Lilly, Pfizer, Calithera Biosciences, Acetylon, Seattle Genetics, Amgen, Zentalis Pharmaceuticals, and CytomX Therapeutics; Speakers Bureau—Eli-Lilly; Spouse—Array Biopharma, Pfizer, Grail.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kearney, M.R., McGuinness, J.E. & Kalinsky, K. Clinical trial data and emerging immunotherapeutic strategies: hormone receptor-positive, HER2− negative breast cancer. Breast Cancer Res Treat 189, 1–13 (2021). https://doi.org/10.1007/s10549-021-06291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06291-8