Abstract
Purpose
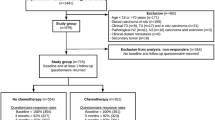
To investigate whether postoperative adjuvant trastuzumab plus chemotherapy negatively affected cognitive functioning during the post-chemotherapy period compared with trastuzumab monotherapy in older patients with HER2-positive breast cancer.
Methods
In the randomized RESPECT trial, women aged between 70 and 80 years with HER2-positive, stage I to IIIA invasive breast cancer who underwent curative operation were randomly assigned to receive either 1-year trastuzumab monotherapy or 1-year trastuzumab plus chemotherapy. Cognitive functioning was assessed using the Mini-Mental State Examination (MMSE) test at enrollment and 1 and 3 years after initiation of the protocol treatment. The primary outcome was change in the MMSE total score from baseline. Secondary outcomes included prevalence of suspected mild cognitive impairment (MMSE total score < 28) and suspected dementia (MMSE total score < 24).
Results
The analytical population consisted of 29 and 26 patients in the trastuzumab monotherapy and trastuzumab plus chemotherapy groups, respectively. The group differences in mean changes of the MMSE total score were 0.6 (95% confidence interval [CI] − 0.3 to 1.6) at 1 year and 0.9 (95% CI − 1.0 to 2.8) at 3 years (P = 0.136 for the group difference pooling the two visits). The prevalence of suspected mild cognitive impairment at 3 years was 41.7% in the trastuzumab monotherapy group and 28.6% in the trastuzumab plus chemotherapy group (P = 0.548).
Conclusion
This randomized sub-study did not show worse cognitive functioning during the post-chemotherapy period with trastuzumab plus chemotherapy than with trastuzumab monotherapy in older patients with HER2-positive breast cancer.
Trial registration number
NCT01104935 (first posted April 16, 2010).



Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
The codes used to analyze the datasets during the current study are available from the corresponding author on reasonable request.
References
Silberfarb PM (1983) Chemotherapy and cognitive defects in cancer patients. Annu Rev Med 34:35–46
Ahles TA, Root JC, Ryan EL (2012) Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 30:3675–3686
Ono M, Ogilvie JM, Wilson JS et al (2015) A meta-analysis of cognitive impairment and decline associated with adjuvant chemotherapy in women with breast cancer. Front Oncol 5:59
Jenkins V, Shilling V, Deutsch G et al (2006) A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer 94:828–834
Hutchinson AD, Hosking JR, Kichenadasse G et al (2012) Objective and subjective cognitive impairment following chemotherapy for cancer: a systematic review. Cancer Treat Rev 38:926–934
Ahles TA, Saykin AJ, Noll WW et al (2003) The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 12:612–619
Mandelblatt JS, Small BJ, Luta G et al (2018) Cancer-related cognitive outcomes among older breast cancer survivors in the thinking and living with cancer study. J Clin Oncol 36:3211–3222
Jim HSL, Phillips KM, Chait S et al (2012) Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol 30:3578–3587
Smith BD, Smith GL, Hurria A et al (2009) Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol 27:2758–2765
Ahles TA, Saykin AJ, McDonald BC et al (2010) Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol 28:4434–4440
Hurria A, Rosen C, Hudis C et al (2006) Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc 54:925–931
Loh KP, Janelsins MC, Mohile SG et al (2016) Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol 7:270–280
Shepard MA (2002) Treatment preferences of seriously ill patients. N Engl J Med 347:533–535
Mandelblatt JS, Jacobsen PB, Ahles T (2014) Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol 32:2617–2626
Sawaki M, Tokudome N, Mizuno T et al (2011) Evaluation of trastuzumab without chemotherapy as a post-operative adjuvant therapy in HER2-positive elderly breast cancer patients: randomized controlled trial [RESPECT (N-SAS BC07)]. Jpn J Clin Oncol 41:709–712
Sawaki M, Taira N, Uemura Y et al (2020) Randomized controlled trial of trastuzumab with or without chemotherapy for HER2-positive early breast cancer in older patients. J Clin Oncol 38:3743–3752
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-Mental State” A practical method for grading the cognitive state of paients for the clinician. J Psychiatr Res 12:189–198
Kaufer DI, Williams CS, Braaten AJ et al (2008) Cognitive screening for dementia and mild cognitive impairment in assisted living: comparison of 3 tests. J Am Med Dir Assoc 9:586–593
Saxton J, Morrow L, Eschman A et al (2009) Computer assessment of mild cognitive impairment. Postgrad Med 121:177–185
Tariq SH, Tumosa N, Chibnall JT et al (2006) Comparison of the Saint Louis University Mental Status examination and the Mini-Mental State Examination for detecting dementia and mild neurocognitive disorder - a pilot study. Am J Geriatr Psychiatry 14:900–910
Tsoi KKF, Chan JYC, Hirai HW et al (2015) Cognitive tests to detect dementia a systematic review and meta-analysis. JAMA Intern Med 175:1450–1458
Wefel JS, Vardy J, Ahles T, Schagen SB (2011) International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol 12:703–708
Wildiers H, Kenis C (2012) Comprehensive geriatric assessment (CGA) in older oncological patients: why and how? J Geriatr Oncol 3:174–176
Rambeau A, Beauplet B, Laviec H et al (2019) Prospective comparison of the Montreal Cognitive Assessment (MoCA) and the Mini Mental State Examination (MMSE) in geriatric oncology. J Geriatr Oncol 10:235–240
Mandelblatt JS, Stern RA, Luta G et al (2014) Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol 32:1909–1918
Extermann M, Hurria A (2007) Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol 25:1824–1831
Schilder CM, Seynaeve C, Beex LV et al (2010) Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol 28:1294–1300
Yamada TH, Denburg NL, Beglinger LJ, Schultz SK (2014) Neuropsychological outcomes of older breast cancer survivors: cognitive features ten or more years after chemotherapy. J Neuropsychiatry Clin Neurosci 22:48–54
Crum RM, Anthony JC, Bassett SS, Folstein MF (1993) Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 269:2386–2391
Acknowledgements
The RESPECT trial was funded by the Comprehensive Support Project for Oncology Research (CSPOR) of the Public Health Research Foundation. We thank all patients who participated in the RESPECT trial and investigators, medical staff, and data managers who were dedicated to this trial. We are grateful for support from the Comprehensive Support Project for Health Outcomes Research (CSP-HOR).
Funding
The RESPECT trial was funded by the Comprehensive Support Project for Oncology Research (CSPOR) of the Public Health Research Foundation.
Author information
Authors and Affiliations
Contributions
Study concept and design: MS, KS, YO, and NT. Administrative support: HM and NT. Provision of study material or patients: MS, YO, MT, SB, HM, and NT. Collection and assembly of data: MS, MT, TS, SB, KK, HM, and NT. Data analysis and interpretation: YH, MS, YU, TK, YO, MT, SB, HM, and NT. Statistical analysis: YH, YU, TK, and YO. Manuscript preparation: YH, MS, and NT. Manuscript editing: YH. Manuscript review: All authors.
Corresponding author
Ethics declarations
Conflict of interest
YU reports honoraria from Chugai Pharma and Teijin Pharma; consulting and advisory role for Pfizer, Ono Pharmaceutical, Zeria Pharmaceutical, and Daiichi Sankyo; speakers' bureaus for Pfizer; and travel support from Pfizer and Chugai Pharma. YO reports compensated leadership for Statcom; stock ownership of Statcom; honoraria from Chugai Pharma, Daiichi Sankyo, Sanofi, Eisai, and Shionogi; and grants from Medical Member System. MT reports honoraria from AstraZeneca, Eli Lilly, Eisai, and Pfizer; and grants paid to the institution from Eisai, Kyowa Kirin, and Taiho Pharmaceutical. HM reports honoraria from Pfizer, Daiichi Sankyo, Taiho Pharmaceutical, and Takeda; and grants paid to the institution from Daiichi Sankyo. All remaining authors have declared no conflicts of interest.
Ethical approval
The RESPECT trial protocol, including the cognitive functioning sub-study, was approved by the institutional review boards of all participating institutions.
Informed consent
All patients provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hagiwara, Y., Sawaki, M., Uemura, Y. et al. Impact of chemotherapy on cognitive functioning in older patients with HER2-positive breast cancer: a sub-study in the RESPECT trial. Breast Cancer Res Treat 188, 675–683 (2021). https://doi.org/10.1007/s10549-021-06253-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06253-0