Abstract
Purpose
Results from TAILOR-X suggest that up to 70% of hormone receptor-positive (HR+) node-negative (N0) ESBC patients (pts) may avoid chemotherapy (CT) with RS ≤ 25. We assess clinical and economic impacts of RS testing on treatment using real-world data.
Methods
From October 2011 to February 2019, a retrospective, cross-sectional observational study was conducted of HR+ N0 ESBC pts who had RS testing in Ireland. Pts were classified low risk (RS ≤ 25) and high risk (RS > 25). Clinical risk was calculated. Data were collected via electronic patient records. Cost data were supplied by the National Healthcare Pricing Regulatory Authority.
Results
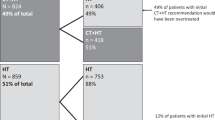
963 pts. Mean age is 56 years. Mean tumour size is 1.7 cm. 114 (11.8%), 635 (66%), 211 (22%), 3 (0.2%) pts had G1, G2, G3 and unknown G, respectively. 796 pts (82.8%) low RS, 159 (16.5%) high RS and 8 pts (0.7%) unknown RS. 263 pts (26%) were aged ≤ 50 at diagnosis; 117 (45%) had RS 0–15, 63 (24.5%) 16–20, 39 (15.3%) 21–25 and 40 (15.2%) RS 26–100. 4 pts (1.5%) had unknown RS. Post-RS testing, 602 pts (62.5%) had a change in CT decision; 593 changed to hormone therapy (HT) alone. In total, 262 pts received CT. Of pts receiving CT; 138 (53%) had RS > 25, 124 (47%) had RS ≤ 25. Of pts aged ≤ 50, 153 (58%) had high clinical risk, of whom 28 had RS 16–20. Assay use achieved a 62.5% change in treatment with 73% of pts avoiding CT. This resulted in savings of €4 million in treatment costs. Deducting assay costs, savings of €1.9 million were achieved.
Conclusion
Over the 8 years of the study, a 62.5% reduction in CT use was achieved with savings of over €1,900,000.
Similar content being viewed by others
Data availability
The fully anonymised datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
NCRI (2019) Cancer in Ireland 1994–2017 with estimates for 2017–2019: annual report of the National Cancer Registry. Cork, Ireland
NCRI (2019) Cancer Trends No. 37. Breast cancer 1994–2016. Cork, Ireland
Dignam JJ et al (2009) Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res Treat 116(3):595–602
Goldvaser H et al (2018) Absolute benefit from adjuvant chemotherapy in contemporary clinical trials: a systemic review and meta-analysis. Cancer Treat Rev 71:68–75
Fisher B et al (2004) Treatment of axillary lymph node-negative, estrogen receptor-negative breast cancer: updated findings from National Surgical Adjuvant Breast and Bowel Project Clinical Trials. J Natl Cancer Inst 96(24):1823–1831
Shapiro CL, Recht A (2001) Side effects of adjuvant treatment of breast cancer. N Engl J Med 344(26):1997–2008
Cardoso F et al (2019) Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 30(8):1194–1220
Harris LN et al (2016) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34(10):1134–1150
Cardoso F et al (2016) 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 375(8):717–729
Cardoso F et al (2020) MINDACT: long-term results of the large prospective trial testing the 70-gene signature MammaPrint as guidance for adjuvant chemotherapy in breast cancer patients. J Clin Oncol 38(15_suppl):506–506
Martin M et al (2014) Clinical validation of the EndoPredict test in node-positive, chemotherapy-treated ER+/HER2− breast cancer patients: results from the GEICAM 9906 trial. Breast Cancer Res 16(2):R38
Dubsky P et al (2013) The EndoPredict score provides prognostic information on late distant metastases in ER+/HER2− breast cancer patients. Br J Cancer 109(12):2959–2964
Filipits M et al (2014) The PAM50 risk-of-recurrence score predicts risk for late distant recurrence after endocrine therapy in postmenopausal women with endocrine-responsive early breast cancer. Clin Cancer Res 20(5):1298–1305
Paik S et al (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
Paik S et al (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734
Xin L et al (2017) The era of multigene panels comes? The clinical utility of Oncotype DX and MammaPrint. World J Oncol 8(2):34–40
Geyer CE et al (2018) 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. NPJ Breast Cancer 4(37)
Albain KS et al (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65
Sparano JA et al (2018) Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 379(2):111–121
Sparano JA et al (2019) Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med 380(25):2395–2405
Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22
Albanell J et al (2016) Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor–positive, human epidermal growth factor receptor 2–negative early-stage breast cancer. Eur J Cancer 66:104–113
Crolley VE et al (2017) The impact of Oncotype DX breast cancer assay results on clinical practice: a UK experience. Ann Oncol 28:v57–v58
Dieci MV et al (2019) Impact of 21-gene breast cancer assay on treatment decision for patients with T1–T3, N0–N1, estrogen receptor-positive/human epidermal growth receptor 2-negative breast cancer: final results of the prospective multicenter ROXANE study. Oncologist 24(11):1424–1431
Lo SS et al (2010) Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J Clin Oncol 28(10):1671–1676
Khan MA et al (2018) The Warwick experience of the Oncotype DX® Breast Recurrence Score® assay as a predictor of chemotherapy administration. Breast Care 13(5):369–372
Klang SH et al (2010) Economic implications of 21-gene breast cancer risk assay from the perspective of an Israeli-Managed Health-Care Organization. Value Health 13(4):381–387
Plun-Favreau J et al (2013) Cost-effectiveness analysis of the use of Oncotype Dx to guide adjuvant chemotherapy decisions in breast cancer patients in Mexico. Value Health 16(3):A140
Yamauchi H et al (2014) Societal cost-effectiveness analysis of the 21-gene assay in estrogen-receptor–positive, lymph-node–negative early-stage breast cancer in Japan. BMC Health Serv Res 14(1):372
Tsoi DT et al (2010) Cost-effectiveness analysis of recurrence score-guided treatment using a 21-gene assay in early breast cancer. Oncologist 15(5):457–465
Harnan S et al (2019) Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer: a systematic review and economic analysis. Health Technol Assess 23:30
Ontario Health (Quality) (2020) Gene expression profiling tests for early-stage invasive breast cancer: a health technology assessment. Ont Health Technol Assess Ser 20(10):1–234
Siow ZR et al (2018) Spotlight on the utility of the Oncotype DX<sup>®</sup> breast cancer assay. Int J Women’s Health 10:89–100
National Centre for Pharmacoeconomics (2011) Cost-effectiveness of Oncotype DX® to target chemotherapy use in lymph-node-negative, oestrogen-receptor-positive, early-stage breast cancer in Ireland. http://www.ncpe.ie/wp-content/uploads/2011/10/Oncotype-DX-summary1.pdf. Accessed 8 Mar 2020
Smyth L et al (2015) Economic impact of 21-gene recurrence score testing on early-stage breast cancer in Ireland. Breast Cancer Res Treat 153(3):573–582
Mook S et al (2009) Calibration and discriminatory accuracy of prognosis calculation for breast cancer with the online Adjuvant! program: a hospital-based retrospective cohort study. Lancet Oncol 10(11):1070–1076
Olivotto IA et al (2005) Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 23(12):2716–2725
Office, H.P. (2019) ABF 2019 admitted patient price list. HSE, Dublin
Oh P-J, Cho J-R (2020) Changes in fatigue, psychological distress, and quality of life after chemotherapy in women with breast cancer. Cancer Nurs 43(1):E54–E60
Reyes SA et al (2019) Practice changing potential of TAILORx: a retrospective review of the National Cancer Data Base from 2010 to 2015. Ann Surg Oncol 26(10):3397–3408
Liu K-H et al (2020) Should women with early breast cancer under 40 years of age have a routine 21-gene recurrence score testing: a SEER database study. Breast 49:233–241
Greally M et al (2019) Where youth matters—clinicopathologic characteristics and emerging trends in treatment and outcomes in young Irish women with breast cancer. Ir J Med Sci 188(1):59–67
Crolley VE et al (2020) The impact of Oncotype DX breast cancer assay results on clinical practice: a UK experience. Breast Cancer Res Treat 180(3):809–817
Rabie MA et al (2019) The effect of Oncotype DX® on adjuvant chemotherapy treatment decisions in early breast cancer. Ann R Coll Surg Engl 101(8):596–601
Torres S et al (2018) Prospective evaluation of the impact of the 21-gene recurrence score assay on adjuvant treatment decisions for women with node-positive breast cancer in Ontario, Canada. Oncologist 23(7):768–775
Friese CR et al (2017) Chemotherapy decisions and patient experience with the recurrence score assay for early-stage breast cancer. Cancer 123(1):43–51
Mariotto A et al (2020) Expected monetary impact of Oncotype DX score-concordant systemic breast cancer therapy based on the TAILORx trial. JNCI J Natl Cancer Inst 112(2):154–160
Wang S-Y et al (2019) Incorporating tumor characteristics to maximize 21-gene assay utility: a cost-effectiveness analysis. J Natl Compr Cancer Netw 17(1):39–46
Kantar (2019) The real cost of cancer. Irish Cancer Society, Dublin. https://www.cancer.ie/sites/default/files/2020-01/Real%20Cost%20of%20Cancer%202019%20report.pdf. Accessed 12 Aug 2020
Kelly CM (2011) Oncotype-DX® gene expression profile and chemotherapy decision-making in patients with early stage breast cancer. In: Evidence-based Series #1. Irish Society of Medical Oncology, Dublin. https://www.hse.ie/eng/services/list/5/cancer/profinfo/chemoprotocols/breast/oncdx.pdf. Accessed 13 Apr 2020
Acknowledgements
Statistical analysis assistance from Kevin O’Riordan is acknowledged.
Funding
Not applicable. No funding received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SJ Millen is an employee of Exact Sciences and provided assistance with budget impact calculations. LM Smyth has the following disclosures: Employment: Loxo Oncology at Lilly; Stock and Other Ownership Interests: Lilly; Honoraria: AstraZeneca, Pfizer, Roche/Genentech; Consulting or Advisory Role: AstraZeneca, Roche/Genentech, Loxo Oncology at Lilly, Pfizer, Novartis; Research Funding: AstraZeneca, Roche/Genentech, Puma Biotechnology; Travel, Accommodations, Expenses: Pfizer, Roche/Genentech, Puma Biotechnology. All remaining authors have declared no conflict of interest.
Ethical approval
This is a retrospective observational study of data obtained for clinical purposes. Ethical approval was not required. The study is registered with and approved by the Clinical Audit Committee at St Vincent’s University Hospital, Dublin 4, Ireland. This research was conducted in accordance with the 1964 Helsinki Declaration and its later amendments, and national and institutional research standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McSorley, L.M., Tharmabala, M., Al Rahbi, F. et al. Real-world analysis of clinical and economic impact of 21-gene recurrence score (RS) testing in early-stage breast cancer (ESBC) in Ireland. Breast Cancer Res Treat 188, 789–798 (2021). https://doi.org/10.1007/s10549-021-06211-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06211-w