Abstract
Purpose
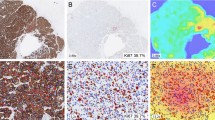
This study aimed to determine the interobserver concordance of two methods for proliferation assessment in breast cancer using Ki67 immunohistochemistry.
Methods
Ki67 was independently assessed in randomly selected tumour samples from patients with lymph node-negative breast cancer using two different methods: either cell counting or visual estimation of hot spot areas. For hot spot cell counting, positive and negative cell numbers were recorded for total cell counts of 300–500, 500–800 and 800–1000 cells. Visual estimation involved allocation of a score from 1 to 5 using a visual scale to estimate percentage positivity. Interobserver agreement for hot spot counting was calculated using a two-way fixed effects intraclass correlation model, and by using Cohen’s kappa measure for visual assessment. Prognostic concordance between the two methods was also calculated using Cohen’s kappa.
Results
Samples from 96 patients were included in this analysis. Interobserver agreement for hot spot cell counting was excellent (> 0.75) across all three cell count ranges, with correlation coefficients of 0.88 (95% CI 0.84–0.92), 0.87 (95% CI 0.82–0.91) and 0.89 (95% CI 0.85–0.92), respectively. Interobserver agreement with visual estimation was greatest for hot spots compared with areas of intermediate or low proliferation, with kappa scores of 0.49, 0.42 and 0.40, respectively. Both assessment methods demonstrated excellent prognostic agreement.
Conclusions
Interobserver and prognostic concordance in Ki67 immunohistochemistry assessments was high using either hot spot cell counting or visual estimation, further supporting the utility and reproducibility of these cost-efficient methods to assess proliferation.



Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Dowsett M, Nielsen TO, A’Hern R et al (2011) Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 103(22):1656–1664
Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, Boisseau M (1991) Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry 12(1):42–49
de Azambuja E, Cardoso F, de Castro G, Jr., Colozza M, Mano MS, Durbecq V, Sotiriou C, Larsimont D, Piccart-Gebhart MJ, Paesmans M, (2007) Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 96(10):1504–1513
Kilickap S, Kaya Y, Yucel B, Tuncer E, Babacan NA, Elagoz S (2014) Higher Ki67 expression is associates with unfavorable prognostic factors and shorter survival in breast cancer. Asian Pac J Cancer Prev 15(3):1381–1385
Fasching PA, Heusinger K, Haeberle L et al (2011) Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 11:486
Polley MY, Leung SC, McShane LM et al (2013) An international Ki67 reproducibility study. J Natl Cancer Inst 105(24):1897–1906
Leung SC, Nielsen TO, Zabaglo L, Arun I, Badve SS, Bane AL, Bartlett JM, Borgquist S, Chang MC, Dodson A (2016) Analytical validation of a standardized scoring protocol for Ki67: phase 3 of an international multicenter collaboration. NPJ Breast Cancer 2:16014
Polley M-YC, Leung SC, Gao D, Mastropasqua MG, Zabaglo LA, Bartlett JM, McShane LM, Enos RA, Badve SS, Bane AL (2015) An international study to increase concordance in Ki67 scoring. Mod Pathol 28(6):778
Hida AI, Bando K, Sugita A et al (2015) Visual assessment of Ki67 using a 5-grade scale (Eye-5) is easy and practical to classify breast cancer subtypes with high reproducibility. J Clin Pathol 68(5):356–361
Abubakar M, Howat WJ, Daley F et al (2016) High-throughput automated scoring of Ki67 in breast cancer tissue microarrays from the Breast Cancer Association Consortium. J Pathol Clin Res 2(3):138–153
Rimm DL, Leung SCY, McShane LM et al (2019) An international multicenter study to evaluate reproducibility of automated scoring for assessment of Ki67 in breast cancer. Mod Pathol 32(1):59–69
Voros A, Csorgo E, Nyari T, Cserni G (2013) An intra- and interobserver reproducibility analysis of the Ki-67 proliferation marker assessment on core biopsies of breast cancer patients and its potential clinical implications. Pathobiology 80(3):111–118
Pathmanathan N, Balleine RL (2013) Ki67 and proliferation in breast cancer. J Clin Pathol 66(6):512–516
Pathmanathan N, Balleine RL, Jayasinghe UW, Bilinski KL, Provan PJ, Byth K, Bilous AM, Salisbury EL, Boyages J (2014) The prognostic value of Ki67 in systemically untreated patients with node-negative breast cancer. J Clin Pathol 67(3):222–228
Boyages J, Taylor R, Chua B, Ung O, Bilous M, Salisbury E, Wilcken N (2006) A risk index for early node-negative breast cancer. Br J Surg 93(5):564–571
Abraira V, de Vargas AP (1999) Generalization of the kappa coeficient for ordinal categorical data, multiple observers and incomplete designs (in Spanish). Qüestiió 23(3):561–571
Cattaneo M, Malighetti P, Spinelli D (2017) Estimating receiver operative characteristic curves for time-dependent outcomes: the stroccurve package. Stand Genomic Sci 17(4):1015–1023
Liu X (2012) Classification accuracy and cut point selection. Stat Med 31(23):2676–2686
Cicchetti DV (1994) Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol Assess 6(4):284
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33(1):159–174
Luporsi E, Andre F, Spyratos F et al (2012) Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat 132(3):895–915
Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M (2013) Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol 24(9):2206–2223
Reyal F, Hajage D, Savignoni A et al (2013) Long-term prognostic performance of Ki67 rate in early stage, pT1-pT2, pN0, invasive breast carcinoma. PLoS ONE 8(3):e55901
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19(5):403–410
Maranta AF, Broder S, Fritzsche C, Knauer M, Thürlimann B, Jochum W, Ruhstaller T (2020) Do YOU know the Ki-67 index of your breast cancer patients? Knowledge of your institution’s Ki-67 index distribution and its robustness is essential for decision-making in early breast cancer. Breast 51:120–126
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826
van Diest PJ, van der Wall E, Baak JP (2004) Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol 57(7):675–681
Leung SCY, Nielsen TO, Zabaglo LA et al (2019) Analytical validation of a standardised scoring protocol for Ki67 immunohistochemistry on breast cancer excision whole sections: an international multicentre collaboration. Histopathology 75(2):225–235
Acknowledgement
Editorial assistance in manuscript preparation was provided by Joyce Lee, PhD, of Nucleus Global, Sydney, Australia.
Funding
This was an investigator-driven study supported by Westmead Breast Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ST declares employment at Australian Clinical Labs, Bella Vista, NSW 2153, Australia (July 2015 to present). BEB declares medical writing fees and statistical consulting fees paid to WriteSource Medical Pty Ltd for services provided to the following pharmaceutical companies: AbbVie, Actelion, Alexion, Bayer, BMS, Ferring, George Clinical, Hartmann, Janssen-Cilag Australia, Lilly, Lundbeck, Medlab, Merck, Novartis, Nucleus Network, Pfizer, Pharmaxis, Roche, Sandoz, Sanofi, UCB. SC declares remuneration from Tissue Pathology and Diagnostic Oncology, ICPMR, Pathology West. HM declares remuneration from the Institute of Clinical Pathology and Medical Research, Pathology West. GF declares remuneration has been received from SA Pathology, Royal Adelaide Hospital. RB declares employment at the Institute of Clinical Pathology and Medical Research, Pathology West, NSW Health Pathology, NSW 2145 (to October 2016) and the Children’s Medical Research Institute, Westmead NSW (October 2016 to present). NP declares remuneration from Douglass Hanly Moir Pathology. The remaining authors have no conflicts to declare.
Ethical approval
Ethics approval was obtained from Westmead Scientific Advisory QA Committee and the Secretary of the WSLHD Human Research Ethics Committee. Reference number: 1812–10 QA.
Consent to participate
This project was approved as a quality assurance project. Consent was not required as data were collected retrospectively, and data were de-identified prior to analysis.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thomas, S., Kabir, M., Butcher, B.E. et al. Interobserver concordance in visual assessment of Ki67 immunohistochemistry in surgical excision specimens from patients with lymph node-negative breast cancer. Breast Cancer Res Treat 188, 729–737 (2021). https://doi.org/10.1007/s10549-021-06188-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06188-6