Abstract
Purpose
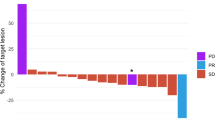
We recently reported results of a phase II trial that camrelizumab plus apatinib induced an objective response rate (ORR) at 43.3% in advanced triple-negative breast cancer (TNBC). This study presents analysis of potential biomarkers.
Methods
TILs, CD8+ T cells and PD-1/PD-L1 expression were evaluated in tumor samples by immunohistochemistry. 59 Cytokines/chemokines, growth factors, or checkpoint-related proteins, blood immune cell subpopulations were analyzed in blood samples by multiplexed bead immunoassays or flow cytometry. Correlation between biomarkers and clinical outcomes including ORR, progression-free survival (PFS), and overall survival (OS) was analyzed.
Results
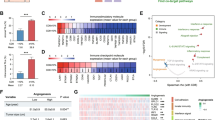
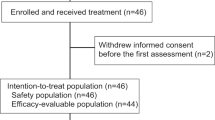
28 Patients had biopsies and blood collected. Baseline TILs were significantly associated with longer PFS (P = 0.035). An increase of tumor-infiltrating CD8+ T cells > 15% during therapy was associated with higher ORR (P = 0.040). Patients with lower baseline plasma levels of HGF or IL-8 were more likely to respond to treatment (P = 0.005 or 0.001, respectively), and showed a longer PFS and OS. Patients with a decrease of IL-8, or an increase of TIM-3 or CD152 during treatment responded more to treatment (P = 0.008, 0.040, or 0.014, respectively). Responders had a higher baseline CD4+ T cells and B cell proportions in blood than non-responders (P = 0.002 and 0.030, respectively).
Conclusion
Higher baseline TILs or a greater increase of tumor-infiltrating CD8+ T cells during therapy, lower baseline plasma HGF/IL-8, a decrease of plasma IL-8, an increase of plasma TIM-3/CD152 during therapy, higher baseline CD4+ T cells or B cells proportion in blood are potential biomarkers for combinational anti-angiogenesis and immunotherapy in advanced TNBC patients.






Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
Consent for publication
Not applicable.
References
Li XX, Yang J, Peng LM, Sahin AA, Huo L, Ward KC, O’Regan R, Torres MA, Meisel JL (2017) Triple-negative breast cancer has worse overall survival and cause-specific survival than non-triple-negative breast cancer. Breast Cancer Res Treat 161:279–287. https://doi.org/10.1007/s10549-016-4059-6
He XX, Ji JL, Dong RR, Liu H, Dai XL, Wang CJ, Esteva FJ, Yeung SCJ (2019) Prognosis in different subtypes of metaplastic breast cancer: a population-based analysis. Breast Cancer Res Treat 173:329–341. https://doi.org/10.1007/s10549-018-5005-6
Malorni L, Shetty PB, De Angelis C, Hilsenbeck S, Rimawi MF, Elledge R, Osborne CK, De Placido S, Arpino G (2012) Clinical and biologic features of triple-negative breast cancers in a large cohort of patients with long-term follow-up. Breast Cancer Res Treat 136:795–804. https://doi.org/10.1007/s10549-012-2315-y
Metzger-Filho O, Sun ZX, Viale G, Price KN, Crivellari D, Snyder RD, Gelber RD, Castiglione-Gertsch M, Coates AS, Goldhirsch A, Cardoso F (2013) Patterns of recurrence and outcome according to breast cancer subtypes in lymph node-negative disease: results from International Breast Cancer Study Group trials VIII and IX. J Clin Oncol 31:3083–3090. https://doi.org/10.1200/JCO.2012.46.1574
Li CY, Zhang S, Zhang XB, Wang P, Hou GF, Zhang J (2013) Clinicopathological and prognostic characteristics of triple-negative breast cancer (TNBC) in Chinese patients: a retrospective study. Asian–Pac J Cancer Prev 14:3779–3784. https://doi.org/10.7314/apjcp.2013.14.6.3779
Qiu J, Xue X, Hu C, Xu H, Kou D, Li R, Li M (2016) Comparison of clinicopathological features and prognosis in triple-negative and non-triple negative breast cancer. J Cancer 7:167–173. https://doi.org/10.7150/jca.10944
Yang Y, Wang Y, Deng H, Tan C, Li Q, He Z, Wei W, Zhou E, Liu Q, Liu J (2019) Development and validation of nomograms predicting survival in Chinese patients with triple negative breast cancer. BMC Cancer 19:541. https://doi.org/10.1186/s12885-019-5703-4
den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA (2017) Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat 161:549–556. https://doi.org/10.1007/s10549-016-4080-9
Gobbini E, Ezzalfani M, Dieras V, Bachelot T, Brain E, Debled M, Jacot W, Mouret-Reynier MA, Goncalves A, Dalenc F, Patsouris A, Ferrero JM, Levy C, Lorgis V, Vanlemmens L, Lefeuvre-Plesse C, Mathoulin-Pelissier S, Petit T, Uwer L, Jouannaud C, Leheurteur M, Lacroix-Triki M, Cleaud AL, Robain M, Courtinard C, Cailliot C, Perol D, Delaloge S (2018) Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur J Cancer 96:17–24. https://doi.org/10.1016/j.ejca.2018.03.015
Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, Kuter I, Nanda R, Cassier PA, Delord JP, Gordon MS, ElGabry E, Chang CW, Sarkar I, Grossman W, O’Hear C, Fasso M, Molinero L, Schmid P (2019) Long-term clinical outcomes and biomarker analyses of atezolizumab therapy for patients with metastatic triple-negative breast cancer: a Phase 1 study. JAMA Oncol 5:74–82. https://doi.org/10.1001/jamaoncol.2018.4224
Nanda R, Chow LQM, Dees EC, Berger R, Gupta S, Geva R, Pusztai L, Pathiraja K, Aktan G, Cheng JD, Karantza V, Buisseret L (2016) Pembrolizumab in patients with advanced triple-negative breast cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol 34:2460–2467. https://doi.org/10.1200/JCO.2015.64.8931
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, Boccia R, Lippman ME, Somer R, Smakal M, Emens LA, Hrinczenko B, Edenfield W, Gurtler J, von Heydebreck A, Grote HJ, Chin K, Hamilton EP (2018) Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: a phase 1b JAVELIN Solid Tumor study. Breast Cancer Res Treat 167:671–686. https://doi.org/10.1007/s10549-017-4537-5
Liu JQ, Liu Q, Li Y, Li Q, Su FX, Yao HR, Su SC, Wang QR, Jin L, Wang Y, Lau WY, Jiang ZF, Song EW (2020) Efficacy and safety of camrelizumab combined with apatinib in advanced triple-negative breast cancer: an open-label phase II trial. J Immunother Cancer. https://doi.org/10.1136/jitc-2020-000696
Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA (2018) Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108–2121. https://doi.org/10.1056/NEJMoa1809615
Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Henschel V, Molinero L, Chui SY, Maiya V, Husain A, Winer EP, Loi S, Emens LA (2020) Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:44–59. https://doi.org/10.1016/S1470-2045(19)30689-8
Neoadjuvant pembrolizumab takes on TNBC (2019). Cancer Discov 9:OF4. https://doi.org/10.1158/2159-8290.CD-NB2019-097
McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM (2006) REporting recommendations for tumor MARKer prognostic studies (REMARK). Breast Cancer Res Treat 100:229–235. https://doi.org/10.1007/s10549-006-9242-8
Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S (2015) The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 26:259–271. https://doi.org/10.1093/annonc/mdu450
Lu ZH, Zou JL, Hu Y, Li S, Zhou T, Gong JF, Li J, Zhang XT, Zhou J, Lu M, Wang XC, Peng Z, Qi CS, Li YY, Li J, Li Y, Zou JY, Du X, Zhang HH, Shen L (2019) Serological markers associated with response to immune checkpoint blockade in metastatic gastrointestinal tract cancer. JAMA Netw Open 2:e197621. https://doi.org/10.1001/jamanetworkopen.2019.7621
Loi S, Giobbie-Hurder A, Gombos A, Bachelot T, Hui R, Curigliano G, Campone M, Biganzoli L, Bonnefoi H, Jerusalem G, Bartsch R, Rabaglio-Poretti M, Kammler R, Maibach R, Smyth MJ, Di Leo A, Colleoni M, Viale G, Regan MM, Andre F (2019) Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b–2 trial. Lancet Oncol 20:371–382. https://doi.org/10.1016/S1470-2045(18)30812-X
Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, Brandes M, Eickhoff S, Das I, Shridhar N, Hinze D, Rogava M, van der Sluis TC, Ruotsalainen JJ, Gaffal E, Landsberg J, Ludwig KU, Wilhelm C, Riek-Burchardt M, Muller AJ, Gebhardt C, Scolyer RA, Long GV, Janzen V, Teng MWL, Kastenmuller W, Mazzone M, Smyth MJ, Tuting T, Holzel M (2017) Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 47:789-802.e789. https://doi.org/10.1016/j.immuni.2017.09.012
Gebhardt C, Sevko A, Jiang HH, Lichtenberger R, Reith M, Tarnanidis K, Holland-Letz T, Umansky L, Beckhove P, Sucker A, Schadendorf D, Utikal J, Umansky V (2015) Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res 21:5453–5459. https://doi.org/10.1158/1078-0432.CCR-15-0676
Peng SL, Wang R, Zhang XJ, Ma YY, Zhong LH, Li K, Nishiyama A, Arai S, Yano S, Wang W (2019) EGFR-TKI resistance promotes immune escape in lung cancer via increased PD-L1 expression. Mol Cancer 18:165. https://doi.org/10.1186/s12943-019-1073-4
Papaccio F, Della Corte CM, Viscardi G, Di Liello R, Esposito G, Sparano F, Ciardiello F, Morgillo F (2018) HGF/MET and the immune system: relevance for cancer immunotherapy. Int J Mol Sci. https://doi.org/10.3390/ijms19113595
Waugh DJJ, Wilson C (2008) The interleukin-8 pathway in cancer. Clin Cancer Res 14:6735–6741. https://doi.org/10.1158/1078-0432.CCR-07-4843
Zarogoulidis P, Katsikogianni F, Tsiouda T, Sakkas A, Katsikogiannis N, Zarogoulidis K (2014) Interleukin-8 and interleukin-17 for cancer. Cancer Investig 32:197–205. https://doi.org/10.3109/07357907.2014.898156
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, Walsh AM, Baxi V, Pandya D, Baradet T, Locke D, Wu QY, Reilly TP, Phillips P, Nagineni V, Gianino N, Gu JL, Zhao HY, Perez-Gracia JL, Sanmamed MF, Melero I (2020) Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med 26:688–692. https://doi.org/10.1038/s41591-020-0856-x
Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, Onate C, Perez G, Alfaro C, Martin-Algarra S, Andueza MP, Gurpide A, Morgado M, Wang J, Bacchiocchi A, Halaban R, Kluger H, Chen L, Sznol M, Melero I (2017) Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol 28:1988–1995. https://doi.org/10.1093/annonc/mdx190
Anderson AC (2014) Tim-3: an emerging target in the cancer immunotherapy landscape. Cancer Immunol Res 2:393–398. https://doi.org/10.1158/2326-6066.CIR-14-0039
Liu QQ, Hu PP, Deng GD, Zhang JX, Liang N, Xie J, Qiao LL, Luo H, Zhang JD (2017) Soluble cytotoxic T-lymphocyte antigen 4: a favorable predictor in malignant tumors after therapy. OncoTargets Ther 10:2147–2154. https://doi.org/10.2147/OTT.S128451
Petitprez F, de Reynies A, Keung EZ, Chen TWW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautes-Fridman C, Tawbi HA, Fridman WH (2020) B cells are associated with survival and immunotherapy response in sarcoma. Nature 577:556–560. https://doi.org/10.1038/s41586-019-1906-8
Helmink BA, Reddy SM, Gao JJ, Zhang SJ, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han GC, Gopalakrishnan V, Xi YX, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu WB, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao DS, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautes-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang LH, Wargo JA (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577:549–555. https://doi.org/10.1038/s41586-019-1922-8
Cabrita R, Lauss M, Sanna A, Donia M, Skaarup LM, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lovgren K, Warren S, Jirstrom K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jonsson G (2020) Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577:561–565. https://doi.org/10.1038/s41586-019-1914-8
Ottonello S, Genova C, Cossu I, Fontana V, Rijavec E, Rossi G, Biello F, Dal Bello MG, Tagliamento M, Alama A, Coco S, Boccardo S, Vanni I, Ferlazzo G, Moretta L, Grossi F, Mingari MC, Carrega P, Pietra G (2020) Association between response to nivolumab treatment and peripheral blood lymphocyte subsets in patients with non-small cell lung cancer. Front Immunol 11:125. https://doi.org/10.3389/fimmu.2020.00125
Subrahmanyam PB, Dong ZW, Gusenleitner D, Giobbie-Hurder A, Severgnini M, Zhou J, Manos M, Eastman LM, Maecker HT, Hodi FS (2018) Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer 6:18. https://doi.org/10.1186/s40425-018-0328-8
Acknowledgements
We thank the patients and their families, nurses, trial coordinators, radiologists and pathologists who participated in this trial.
Funding
This work was supported by Grants from Natural Science Foundation of China Grants (81630074, 81872141), Guangzhou Science and Technology Plan Key Projects (201804020076) and the Fundamental Research Funds for the Central Universities (20ykpy101).
Author information
Authors and Affiliations
Contributions
Conceptualization, QL; Formal analysis, JL, YL and QL; Methodology, JL, YL and QL; Project administration, QL; Writing: original draft, JL, YL and QL; Writing: review and editing, JL, YL, QL, DL, QW and QL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Research involving human participants and/or animals
This research was approved by the Clinical Research Ethics Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University. And all the studies were performed in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS).
Informed consent
Written informed consents were obtained from the participants before sampling.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10549_2021_6128_MOESM1_ESM.tif
(TIF 132 kb) Supplementary Fig. S1 Changes of proportions of blood immune cells and associations of baseline bloodimmune cell subpopulations and clinical outcomes. a CD4+ T cells change during treatment, b overallsurvival stratified by baseline CD4+ T cells level, c B cells change during treatment, and d progression-freesurvival stratified by baseline B cells level
10549_2021_6128_MOESM2_ESM.tif
(TIF 3542 kb) Supplementary Fig. S2 Changes of proportions of blood immune cell subpopulations during treatment. aNK cells, b PD-1+CD4+ T cells, and c PD-1+CD8+ T cells
Rights and permissions
About this article
Cite this article
Liu, J., Li, Y., Li, Q. et al. Biomarkers of response to camrelizumab combined with apatinib: an analysis from a phase II trial in advanced triple-negative breast cancer patients. Breast Cancer Res Treat 186, 687–697 (2021). https://doi.org/10.1007/s10549-021-06128-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06128-4