Abstract
Purpose
Different tumor-related factors have been proposed to assess the risk of disease progression and death in women undergoing neoadjuvant breast cancer chemotherapy. Recently, besides the classical pre-treatment clinical stage (CS) and post-treatment pathologic stage (PS), estrogen receptor status and histologic grade (CPS + EG score) and HER2 results (Neo-Bioscore) have also been added to this suite of staging systems, generating new scores. The present study aims to compare the performance of these four staging systems, namely CS, PS, CPS + EG and Neo-Bioscore, in the prognosis of breast cancer in women undergoing neoadjuvant chemotherapy.
Methods
This study comprises a retrospective cohort study of female breast cancer patients diagnosed at the Brazilian National Cancer Institute, Brazil from January 2013 to December 2015. A descriptive analysis of patient characteristics was conducted, and Kaplan–Meier curves, a Cox proportional hazard analysis and Receiver Operating Characteristic (ROC) curves were developed according to the assessed staging system scores.
Results
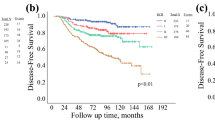
A total of 803 patients were eligible for this study. Most were under 65 years old (88.0%), presented advanced tumors (clinical stage ≥ IIB 77.1%), with positive estrogen receptor (71.2%) and negative HER2 (75.7%) results. During the follow-up, 172 patients (21.4%) evolved to death. A statistical difference (p < 0.001) was observed between 5 year disease-free survival and 5 year overall survival rates according to the PS, CPS + EG and Neo-Bioscore staging systems.
Conclusion
The PS, CPS + EG and Neo-Bioscore staging systems were proven to be equivalent to predict the prognosis of patients undergoing neoadjuvant chemotherapy.

Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to personal nature of the information included, but are available from the corresponding author on reasonable request.
References
Jeruss JS, Mittendorf EA, Tucker SL, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, Cormier JN, Buzdar AU, Hortobagyi GN, Hunt KK (2008) Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol 26(2):246–252. https://doi.org/10.1200/JCO.2007.11.5352
Mittendorf EA, Jeruss JS, Tucker SL, Kolli A, Newman LA, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, Cormier JN, Buzdar AU, Hortobagyi GN, Hunt KK (2011) Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 29(15):1956–1962. https://doi.org/10.1200/JCO.2010.31.8469
Michel LL, Sommer L, González Silos R, Lorenzo Bermejo J, von Au A, Seitz J, Hennigs A, Smetanay K, Golatta M, Heil J, Schütz F, Sohn C, Schneeweiss A, Marmé F (2019) Locoregional risk assessment after neoadjuvant chemotherapy in patients with primary breast cancer: clinical utility of the CPS + EG score. Breast Cancer Res Treat 177(2):437–446. https://doi.org/10.1007/s10549-019-05314-9
Nguyen D, Yu J, Reinhold WC, Yang SX (2020) Association of independent prognostic factors and treatment modality with survival and recurrence outcomes in breast cancer. JAMA Netw Open 3(7):e207213. https://doi.org/10.1001/jamanetworkopen.2020.7213
Giuliano AE, Edge SB, Hortobagyi GN (2018) Eighth edition of the AJCC cancer staging manual: breast cancer. Ann Surg Oncol 25(7):1783–1785. https://doi.org/10.1245/s10434-018-6486-6
Medeiros GC, Bergmann A, Aguiar SS, Thuler LC (2015) Análise dos determinantes que influenciam o tempo para o início do tratamento de mulheres com câncer de mama no Brasil [determinants of the time between breast cancer diagnosis and initiation of treatment in Brazilian women]. Cad Saude Publica 31(6):1269–1282. https://doi.org/10.1590/0102-311X00048514
Medeiros GC, Thuler LCS, Bergmann A (2019) Delay in breast cancer diagnosis: a Brazilian cohort study. Public Health 167:88–95. https://doi.org/10.1016/j.puhe.2018.10.012
Medina JD, de Araujo TI, Mendes GN, Silva JG, da Silva Paiva MA, de Aguiar SS, Thuler LC, Bergmann A (2018) Advanced clinical stage at diagnosis of breast cancer is associated with poorer health-related quality of life: a cross-sectional study. Eur J Breast Health 15(1):26–31. https://doi.org/10.5152/ejbh.2018.4297
American Joint Committee on Cancer (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Xu L, Zhang Z, Liu Q, Zhou B, Liu Y, Xiang Q, Zhu S, Duan X, Cui Y (2018) Validation of CPS+EG, neo-bioscore, and modified neo-bioscore staging systems after preoperative systemic therapy of breast cancer: protocol of a retrospective multicenter cohort study in China. Thorac Cancer 9(11):1565–1572. https://doi.org/10.1111/1759-7714.12852
Bursac Z, Gauss CH, Williams DK, Hosmer DW (2008) Purposeful selection of variables in logistic regression. Source Code Biol Med 16(3):17. https://doi.org/10.1186/1751-0473-3-17
Abdelsattar JM, Al-Hilli Z, Hoskin TL, Heins CN, Boughey JC (2016) Validation of the CPS + EG staging system for disease-specific survival in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 23(10):3206–3211. https://doi.org/10.1245/s10434-016-5324-y
Marmé F, Lederer B, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Gerber B, Hanusch C, Hilfrich J, Huober J, Jackisch C, Kümmel S, Loibl S, Paepke S, Untch M, von Minckwitz G, Schneeweiss A (2016) Utility of the CPS+EG staging system in hormone receptor-positive, human epidermal growth factor receptor 2-negative breast cancer treated with neoadjuvant chemotherapy. Eur J Cancer 53:65–74. https://doi.org/10.1016/j.ejca.2015.09.022
Xu L, Duan X, Zhou B, Liu Y, Ye J, Liu Z, Ma C, Zhang H, Zhang S, Zhang L, Zhao J, Cheng Y (2018) Validation of the CPS+EG and neo-bioscore staging systems after preoperative systemic therapy for breast cancer in a single center in China. Breast 40:29–37. https://doi.org/10.1016/j.breast.2018.03.010
Goorts B, van Nijnatten TJ, de Munck L, Moossdorff M, Heuts EM, de Boer M, Lobbes MB, Smidt ML (2017) Clinical tumor stage is the most important predictor of pathological complete response rate after neoadjuvant chemotherapy in breast cancer patients. Breast Cancer Res Treat 163(1):83–91. https://doi.org/10.1007/s10549-017-4155-2
Mittendorf EA, Vila J, Tucker SL, Chavez-MacGregor M, Smith BD, Symmans WF, Sahin AA, Hortobagyi GN, Hunt KK (2016) The neo-bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol 2(7):929–936. https://doi.org/10.1001/jamaoncol.2015.6478
Gianni L, Pienkowski T, Im YH, Tseng LM, Liu MC, Lluch A, Starosławska E, de la Haba-Rodriguez J, Im SA, Pedrini JL, Poirier B, Morandi P, Semiglazov V, Srimuninnimit V, Bianchi GV, Magazzù D, McNally V, Douthwaite H, Ross G, Valagussa P (2016) 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol 17(6):791–800. https://doi.org/10.1016/S1470-2045(16)00163-7
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the INCA Research Ethics Committee on December 10, 2012 (CAAE 06794512.3.0000.5274; opinion 166.838).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soares, I.C.S., Bello, M.A., Bergmann, A. et al. Comparison of the performance of four staging systems in determining the prognosis of breast cancer among women undergoing neoadjuvant chemotherapy. Breast Cancer Res Treat 187, 547–555 (2021). https://doi.org/10.1007/s10549-020-06077-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-06077-4