Abstract
Purpose
To find a group of cN2 patients or patients with high axillary burden who become ypN0 after neoadjuvant chemotherapy (NACT) and who may benefit from avoiding a lymphadenectomy.
Methods
A retrospective observational cohort study was conducted with 221 clinically staged N2 patients or patients with at least 3 suspicious lymph nodes found by ultrasound at diagnosis. The predictive factors for ypN0 analysed were age, MRI-determined tumour size, histological subtype, the Nottingham histologic grade, surrogate molecular subtype, ki-67 and vascular invasion when present. Clinical and radiological responses after NACT were also evaluated. Univariate and multivariate analyses by logistic regression were performed. Distant disease-free survival (DDFS) was calculated in relation to the status of the axillary lymph nodes after NACT.
Results
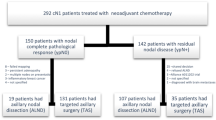
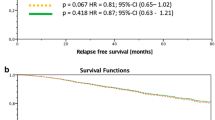
After NACT, 89 patients (40.3%) had axillary pathologic complete response (pCR) (ypN0) and 132 (59.7%) had residual axillary disease (ypN+). Molecular surrogate subtype, Ki-67 expression, and the clinical and radiological responses to NACT were the only independent factors associated with ypN0. Axillary pCR was observed more often in HER2-positive and triple-negative tumours than in luminal ones (OR 7.5 and 3.6, respectively). DDFS was 88.7% (95% CI 80.7–96.7%) for ypN0 and 56.2% (95% CI 32.1–80.3%) for ypN+ (p = 0.09).
Conclusions
In HER2-positive and triple-negative breast cancer patients staged as cN2 or with high axillary burden before NACT, a sentinel lymph node biopsy after NACT could be recommended if there is a clinical and radiological response.


Similar content being viewed by others
References
Cavanaugh KM (2011) Effects of early exercise on the development of lymphedema in patients with breast cancer treated with axillary lymph node dissection. J Oncol Pract 7(2):89–93
Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ et al (2016) Improved axillary evaluation following neoadjuvant therapy for patientswith node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol 34(10):1072–1078
Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang-Köbrunner SH et al (2006) In: Proceedings of the consensus conference on breast conservation, April 28 to May 1, 2005, Milan, Italy. Cancer 107(2):242–250. http://www.ncbi.nlm.nih.gov/pubmed/16770785. Accessed 2 Oct 2014
Veronesi U, Paganelli G, Viale G et al (2003) A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med 349(6):546–553
Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP et al (2010) Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol 11(10):927–933. https://doi.org/10.1016/S1470-2045(10)70207-2
Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B et al (2005) Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 23(12):2694–2702
Takahashi M, Jinno H, Hayashida T, Sakata M, Asakura K, Kitagawa Y (2012) Correlation between clinical nodal status and sentinel lymph node biopsy false negative rate after neoadjuvant chemotherapy. World J Surg 36(12):2847–2852
Alvarado R, Yi M, Le-Petross H, Gilcrease M, Mittendorf EA, Bedrosian I et al (2012) The role for sentinel lymph node dissection after neoadjuvant chemotherapy in patients who present with node-positive breast cancer. Ann Surg Oncol 19(10):3177–3184
Classe JM, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J et al (2009) Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: results of ganglion sentinelle et chimiothérapie neoadjuvante, a French prospective multicentric study. J Clin Oncol 27(5):726–732
Newman EA, Sabel MS, Nees AV, Schott A, Diehl KM, Cimmino VM et al (2007) Sentinel lymph node biopsy performed after neoadjuvant chemotherapy is accurate in patients with documented node-positive breast cancer at presentation. Ann Surg Oncol 14(10):2946–2952
Balch GC, Mithani SK, Richards KR, Beauchamp RD, Kelley MC (2003) Lymphatic mapping and sentinel lymphadenectomy after preoperative therapy for stage II and III breast cancer. Ann Surg Oncol 10(6):616–621
Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL et al (2014) Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 32(13):1365–1383
Boileau J-F, Poirier B, Basik M, Holloway CMB, Gaboury L, Sideris L et al (2014) Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 32:1–9
Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA 310(14):1455–1461
Marc J, Cecile C, Gimbergues LP, Tunon SAC, Dupre DLPF, Rouzier R (2019) Sentinel lymph node biopsy without axillary lymphadenectomy after neoadjuvant chemotherapy is accurate and safe for selected patients : the GANEA 2 study. Breast Cancer Res Treat 173(2):343–352. https://doi.org/10.1007/s10549-018-5004-7
Straver ME, Loo CE, Alderliesten T, Rutgers EJT, Vrancken Peeters MTFD (2010) Marking the axilla with radioactive iodine seeds (MARI procedure) may reduce the need for axillary dissection after neoadjuvant chemotherapy for breast cancer. Br J Surg 97(8):1226–1231
Burstein HJ, Curigliano G, Loibl S, Dubsky P, Gnant M, Poortmans P et al (2019) Estimating the benefits of therapy for early-stage breast cancer : the St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 30(10):1541–1557. https://doi.org/10.1093/annonc/mdz235
Perou CM, Sùrlie T, Eisen MB, Van De Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 533(May):747–752
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K et al (2005) Human cancer biology breast cancer molecular subtypes respond differently to preoperative chemotherapy. Hum Cancer Biol 11(16):5678–5686
Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N et al (2014) Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384(9938):164–172. https://doi.org/10.1016/S0140-6736(13)62422-8
Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL et al (2005) Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol 23(16):3676–3685
Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER. Lancet 375(9712):377–384. https://doi.org/10.1016/S0140-6736(09)61964-4
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32. https://doi.org/10.1016/S1470-2045(11)70336-9
Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM et al (2015) Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603. J Clin Oncol 33(1):13–21
Von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M et al (2014) Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 15(7):747–756
Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J et al (2020) Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382(9):810–821
Fernandez-Gonzalez S, Falo C, Pla MJ, Verdaguer P, Nuñez D, Guma A et al (2020) Predictive factors for omitting lymphadenectomy in patients with node-positive breast cancer treated with neo-adjuvant systemic therapy. Breast J 26(5):888–896
Johnston S, Puhalla S, Wheatley D, Ring A, Barry P, Holcombe C et al (2019) Randomized phase II study evaluating palbociclib in addition to letrozole as neoadjuvant therapy in estrogen receptor–positive early breast cancer: pallet trial. J Clin Oncol 37(3):178–189
Ellis MJ, Suman VJ, Hoog J, Lin L, Snider J, Prat A et al (2011) Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: clinical and biomarker outcomes and predictive value of the baseline PAM50-based int. J Clin Oncol 29(17):2342–2349
Tirkes T, Hollar MA, Tann M, Kohli MD, Akisik F, Sandrasegaran K (2013) Response criteria in oncologic imaging: review of traditional and new criteria. Radiographics 33(5):1323–1341
McCready DR, Yong WS, Ng AKT, Miller N, Done S, Youngson B (2004) Influence of the new AJCC breast cancer staging system on sentinel lymph node positivity and false-negative rates. J Natl Cancer Inst 96(11):873–875
Bedi DG, Krishnamurthy R, Krishnamurthy S, Edeiken BS, Le-Petross H, Fornage BD et al (2008) Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: vitro sonographic study. Am J Roentgenol 191(3):646–652. https://doi.org/10.2214/AJR.07.2460
Wolff AC, McShane LM, Hammond MEH, Allison KH, Fitzgibbons P, Press MF et al (2018) Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med 142(11):1364–1382
Symmans WF, Wei C, Gould R, Yu X, Zhang Y, Liu M et al (2017) Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol 35(10):1049–1060
Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG et al (2014) Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg 260(4):608–614
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321(3):288–300
Kim JY, Park HS, Kim S, Ryu J, Park S, Kim SIL (2015) Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Medicine 94(43):1–9
Mamtani A, Barrio AV, King TA, Van Zee KJ, Plitas G, Pilewskie M et al (2016) How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol 23(11):3467–3474
Diego EJ, McAuliffe PF, Soran A, McGuire KP, Johnson RR, Bonaventura M et al (2016) Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol 23(5):1549–1553
Gillespie TC, Sayegh HE, Brunelle CL, Daniell KM, Taghian AG (2018) Breast cancer-related lymphedema: risk factors, precautionary measures, and treatments. Gland Surg 7(4):379–403
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K et al (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11(16):5678–5685
Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 14(7):609–618
Enokido K, Watanabe C, Nakamura S, Ogiya A, Osako T, Akiyama F et al (2016) Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with an initial diagnosis of cytology-proven lymph node-positive breast cancer. Clin Breast Cancer 16(4):299–304
Pilewskie M, Morrow M (2017) Axillary nodal management following neoadjuvant chemotherapy: a review. JAMA Oncol 3(4):549–555
Banys-Paluchowski M, Gruber IV, Hartkopf A, Paluchowski P, Krawczyk N, Marx M et al (2020) Axillary ultrasound for prediction of response to neoadjuvant therapy in the context of surgical strategies to axillary dissection in primary breast cancer: a systematic review of the current literature. Arch Gynecol Obstet 301(2):341–353. https://doi.org/10.1007/s00404-019-05428-x
Kim R, Chang JM, Lee HB, Lee SH, Kim SY, Kim ES et al (2019) Predicting axillary response to neoadjuvant chemotherapy: breast MRI and US in patients with node-positive breast cancer. Radiology 293(1):49–57
Rodenhuis S, Mandjes IAM, Wesseling J, van de Vijver MJ, Peeters MJTDFV, Sonke GS et al (2009) A simple system for grading the response of breast cancer to neoadjuvant chemotherapy. Ann Oncol 21(3):481–487. https://doi.org/10.1093/annonc/mdp348
Acknowledgements
Michael Maudsley for language revision.
Funding
Not applicable.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that we have no conflict of interest. The authors alone are responsible for the content and writing of the paper.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As a retrospective study, the patient did not receive any specific informed consent for the study. The patients signed the informed consent for the usual procedure.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Garcia-Tejedor, A., Fernandez-Gonzalez, S., Ortega, R. et al. Can we avoid axillary lymph node dissection in N2 breast cancer patients with chemo-sensitive tumours such as HER2 and TNBC?. Breast Cancer Res Treat 185, 657–666 (2021). https://doi.org/10.1007/s10549-020-05970-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05970-2