Abstract
Purpose
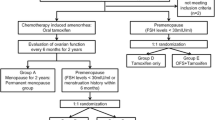
To determine the longitudinal impact of adjuvant chemotherapy and tamoxifen-only treatments on the reproductive potential of women with breast cancer by using a sensitive ovarian reserve marker anti-Mullerian hormone (AMH) as a surrogate.
Methods
One-hundred-and-forty-two women with a primary diagnosis of breast cancer were prospectively followed with serum AMH assessments before the initiation, and 12, 18 and 24 months after the completion of adjuvant chemotherapy or the start of tamoxifen-only treatment. The chemotherapy regimens were classified into Anthracycline-Cyclophosphamide-based (AC-based) and Cyclophosphamide-Methotrexate + 5-Fluorouracil (CMF). Longitudinal data were analyzed by mixed effects model for treatment effects over time, adjusting for baseline age and BMI.
Results
Both chemotherapy regimens resulted in significant decline in ovarian reserve compared to the tamoxifen-only treatment (p < 0.0001 either regimen vs. tamoxifen for overall trend). AMH levels sharply declined at 12 months but did not show a significant recovery from 12 to 18 and 18 to 24 months after the completion of AC-based or CMF regimens. The degree of decline did not differ between the two chemotherapy groups (p = 0.53). In contrast, tamoxifen-only treatment did not significantly alter the age-adjusted serum AMH levels over the 24-month follow up. Likewise, the use of adjuvant tamoxifen following AC-based regimens did not affect AMH recovery.
Conclusions
Both AC-based regimens and CMF significantly compromise ovarian reserve, without a recovery beyond 12 months post-chemotherapy. In contrast, tamoxifen-only treatment does not seem to alter ovarian reserve. These data indicate that the commonly used chemotherapy regimens but not the hormonal therapy compromise future reproductive potential.



Similar content being viewed by others
References
Héry C, Ferlay J, Boniol M, Autier P (2008) Changes in breast cancer incidence and mortality in middle-aged and elderly women in 28 countries with Caucasian majority populations. Ann Oncol 19:1009–1018
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Bedoschi G, Navarro PA, Oktay K (2016) Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol 12:2333–2344
Oktem O, Oktay K (2007) Quantitative assessment of the impact of chemotherapy on ovarian follicle reserve and stromal function. Cancer 110:2222–2229
Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K (2011) Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging 3:782–793
Tham Y-L, Sexton K, Weiss H, Elledge R, Friedman LC, Kramer R (2007) The rates of chemotherapy-induced amenorrhea in patients treated with adjuvant doxorubicin and cyclophosphamide followed by a taxane. Am J Clin Oncol 30:126–132
Oktay KH, Bedoschi G, Goldfarb SB, Taylan E, Titus S, Palomaki GE et al (2020) Increased chemotherapy-induced ovarian reserve loss in women with germline BRCA mutations due to oocyte deoxyribonucleic acid double strand break repair deficiency. Fertil Steril 113:1251–1260
Turan V, Oktay K (2020) BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum Reprod Update 26:43–57
Dunlop CE, Anderson RA (2015) Uses of anti-Mullerian hormone (AMH) measurement before and after cancer treatment in women. Maturitas 80:245–250
Durlinger ALL, Visser JA, Themmen APN (2002) Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction 124:601–609
Fleming R, Seifer DB, Frattarelli JL, Ruman J (2015) Assessing ovarian response: antral follicle count versus anti-Mullerian hormone. Reprod Biomed Online 31:486–496
Hansen KR, Hodnett GM, Knowlton N, Craig LB (2011) Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril 95:170–175
Iliodromiti S, Anderson RA, Nelson SM (2015) Technical and performance characteristics of anti-Mullerian hormone and antral follicle count as biomarkers of ovarian response. Hum Reprod Update 21:698–710
Van Rooij IAJ, Broekmans FJM, Scheffer GJ, Looman CWN, Habbema JF, de Jong FH et al (2005) Serum antiMullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. Fertil Steril 83:979–987
Bentzen JG, Forman JL, Johannsen TH, Pinborg A, Larsen EC, Andersen AN et al (2013) Ovarian antral follicle subclasses and anti-Mullerian hormone during normal reproductive aging. J Clin Endocrinol Metab 98:1602–1611
Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K et al (2013) Which follicles make the most anti-Müllerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod 19:519–527
Durlinger ALL, Gruijters MJG, Kramer P, Karels B, Kumar TR, Matzuk MM et al (2001) Anti-Mullerian hormone attenuates the effects of FSH on follicle development in the mouse ovary. Endocrinology 142:4891–4899
Durlinger ALL, Gruijters MJG, Kramer P, Karels B, Ingraham HA, Nachtigal MW et al (2002) Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology 140:5789–5796
Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R et al (2014) The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update 20:370–385
Riggs R, Kimble T, Oehninger S, Bocca S, Zhao Y, Leader B et al (2011) Anti-Mullerian hormone serum levels predict response to controlled ovarian hyperstimulation but not embryo quality or pregnancy outcome in oocyte donation. Fertil Steril 95:410–412
Riggs RM, Duran EH, Baker MW, Kimble TD, Hobeika E, Yin L et al (2008) Assessment of ovarian reserve with anti-Mullerian hormone: a comparison of the predictive value of anti-Mullerian hormone, follicle-stimulating hormone, inhibin B, and age. Am J Obstet Gynecol 199(202):e1–8
Broer SL, Eijkemans MJC, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP et al (2011) Anti-Mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab 96:2532–2539
Freeman EW, Sammel MD, Lin H, Gracia CR (2012) Anti-Mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab 97:1673–1680
Tehrani FR, Solaymani-Dodaran M, Tohidi M, Gohari MR, Azizi F (2013) Modeling age at menopause using serum concentration of anti-mullerian hormone. J Clin Endocrinol Metab 98:729–735
Anderson RA, Cameron DA (2011) Pretreatment serum anti-Mullerian hormone predicts long-term ovarian function and bone mass after chemotherapy for early breast cancer. J Clin Endocrinol Metab 96:1336–1343
Henry NL, Xia R, Schott AF, McConnell D, Banerjee M, Hayes DF (2014) Prediction of postchemotherapy ovarian function using markers of ovarian reserve. Oncologist 19:68–74
Anderson RA, Themmen APN, Al-Qahtani A, Groome NP, Cameron DA (2006) The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod 21:2583–2592
Lee S, Ozkavukcu S, Heytens E, Moy F, Oktay K (2010) Value of early referral to fertility preservation in young women with breast cancer. J Clin Oncol 28:4683–4686
Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J et al (2010) Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer 116:592–599
Oktay K, Harvey BE, Partridge AH, Quinn GP, Reinecke J, Taylor HS et al (2018) Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 36:1994–2001
Lambertini M, Anserini P, Levaggi A, Poggio F, Del Mastro L (2013) Fertility counseling of young breast cancer patients. J Thorac Dis 5:68–80
Fitzmaurice G, Laird N, Ware J (2011) Longitudinal analysis. 2nd edn, Wiley, Hoboken. https://content.sph.harvard.edu/fitzmaur/ala2e/
Partridge AH, Ruddy KJ, Gelber S, Schapira L, Abusief M, Meyer M et al (2010) Ovarian reserve in women who remain premenopausal after chemotherapy for early stage breast cancer. Fertil Steril 94:638–644
Shandley LM, Spencer JB, Fothergill A, Mertens AC, Manatunga A, Paplomata E et al (2017) Impact of tamoxifen therapy on fertility in breast cancer survivors. Fertil Steril 107:243–252.e5
Oktay K, Buyuk E, Davis O, Yermakova I, Veeck L, Rosenwaks Z (2003) Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum Reprod 18:90–95
Yu B, Douglas N, Ferin MJ, Nakhuda GS, Crew K, Lobo RA et al (2010) Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer 116:2099–2105
Trapp E, Steidl J, Rack B, Kupka MS, Andergassen U, Jückstock J et al (2017) Anti-Müllerian hormone (AMH) levels in premenopausal breast cancer patients treated with taxane-based adjuvant chemotherapy: a translational research project of the SUCCESS A study. Breast 35:130–135
Oktay K, Newton H, Mullan J, Gosden RG (1998) Development of human primordial follicles to antral stages in SCID/hpg mice stimulated with follicle stimulating hormone. Hum Reprod 13:1133–1138
Bines J, Oleske DM, Cobleigh MA (1996) Ovarian function in premenopausal women treated with adjuvant chemotherapy for breast cancer. J Clin Oncol 14:1718–1729
Li F, Turan V, Lierman S, Cuvelier C, De Sutter P, Oktay K (2014) Sphingosine-1-phosphate prevents chemotherapy-induced human primordial follicle death. Hum Reprod 29:107–113
Plante BJ, Cooper GS, Baird DD, Steiner AZ (2010) The impact of smoking on anti-Mullerian hormone levels in women aged 38 to 50 years. Menopause 17:571–576
Acknowledgements
We thank Ansh Laboratories for their assistance in the analysis of serum AMH levels and Angelena Crown, MD for help with data extraction.
Funding
This study was supported by RO1 HD053112 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and National Cancer Institute. H. Bang is partly supported by the National Institutes of Health through Grant UL1 TR001860.
Author information
Authors and Affiliations
Contributions
Conception of the idea: KO; Design: KO, MD, SG, SP; Study execution: SG, MD, KO, GB, VT, ET; Provision of study materials: KO, MD, SG, TC; Manuscript writing: KO, VT, SG, GB, HB; Statistical Analysis: VT, HB; Final approval: All authors.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goldfarb, S.B., Turan, V., Bedoschi, G. et al. Impact of adjuvant chemotherapy or tamoxifen-alone on the ovarian reserve of young women with breast cancer. Breast Cancer Res Treat 185, 165–173 (2021). https://doi.org/10.1007/s10549-020-05933-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05933-7