Abstract
Purpose
To assess the prognostic risk factors and establish prognostic nomograms based on lymph node ratio (LNR) to predict the survival of young patients with breast cancer (BC).
Methods
Patients aged < 40 years and diagnosed with BC between 2010 and 2016 from the Surveillance, Epidemiology and End Results database were assessed. Nomograms incorporating LNR were constructed to predict overall survival (OS) and breast cancer-specific survival (BCSS) based on Cox proportional hazards model. The performance of the nomograms was assessed by C-index, calibration curves, receiver operating characteristic (ROC) curves, decision curve analysis (DCA), and risk group stratification and compared with the TNM staging system.
Results
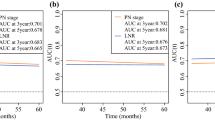
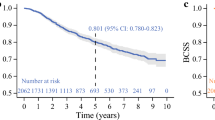
Based on the univariate and multivariate Cox regression analysis, significant prognostic factors were identified and integrated to create the nomograms for OS and BCSS. The calibration curves indicated optimal agreement between model predictions and actual observations. The nomograms showed favorable sensitivity with a C-index of 0.8351 (95% CI 0.8234–0.8469) for OS and 0.8474 (95% CI 0.8355–0.8594) for BCSS. The ROC curves of the nomograms showed better predictive ability than those of the TNM staging system for OS (AUC: 0.8503 vs. 0.7819) and BSCC (AUC: 0.8607 vs. 0.8081). Significant differences in Kaplan–Meier curves were observed in patients stratified into different risk groups (p < 0.001).
Conclusions
These nomograms provided more accurate individualized risk prediction of OS and BCSS and may assist clinicians in making decisions for young patients with BC.






Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA 68(6):394–424. https://doi.org/10.3322/caac.21492
Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, Gelmon K, Gentilini O, Harbeck N, Margulies A, Meirow D, Pruneri G, Senkus E, Spanic T, Sutliff M, Travado L, Peccatori F, Cardoso F (2017) ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast (Edinburgh, Scotland) 35:203–217. https://doi.org/10.1016/j.breast.2017.07.017
Fidler MM, Gupta S, Soerjomataram I, Ferlay J, Steliarova-Foucher E, Bray F (2017) Cancer incidence and mortality among young adults aged 20–39 years worldwide in 2012: a population-based study. Lancet Oncol 18(12):1579–1589. https://doi.org/10.1016/s1470-2045(17)30677-0
Althuis MD, Brogan DD, Coates RJ, Daling JR, Gammon MD, Malone KE, Schoenberg JB, Brinton LA (2003) Breast cancers among very young premenopausal women (United States). Cancer Causes Control 14(2):151–160. https://doi.org/10.1023/A:1023006000760
Maishman T, Cutress RI, Hernandez A, Gerty S, Copson ER, Durcan L, Eccles DM (2017) Local recurrence and breast oncological surgery in young women with breast cancer: the posh observational cohort study. Ann Surg 266(1):165–172. https://doi.org/10.1097/sla.0000000000001930
Peto J, Collins N, Barfoot R, Seal S, Warren W, Rahman N, Easton DF, Evans C, Deacon J, Stratton MR (1999) Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst 91(11):943–949. https://doi.org/10.1093/jnci/91.11.943
Group ABCS (2000) Prevalence and penetrance of BRCA1 and BRCA2 mutations in a population-based series of breast cancer cases. Br J Cancer 83(10):1301–1308. https://doi.org/10.1054/bjoc.2000.1407
Fredholm H, Magnusson K, Lindström LS, Garmo H, Fält SE, Lindman H, Bergh J, Holmberg L, Pontén F, Frisell J, Fredriksson I (2016) Long-term outcome in young women with breast cancer: a population-based study. Breast Cancer Res Treat 160(1):131–143. https://doi.org/10.1007/s10549-016-3983-9
Rudat V, El-Sweilmeen H, Fadel E, Brune-Erber I, Ahmad Nour A, Bushnag Z, Masri N, Altuwaijri S (2012) Age of 40 years or younger is an independent risk factor for locoregional failure in early breast cancer: a single-institutional analysis in saudi arabia. J Oncol 2012:370385. https://doi.org/10.1155/2012/370385
Bleyer A, Barr R, Hayes-Lattin B, Thomas D, Ellis C, Anderson B (2008) The distinctive biology of cancer in adolescents and young adults. Nat Rev Cancer 8(4):288–298. https://doi.org/10.1038/nrc2349
Anders CK, Hsu DS, Broadwater G, Acharya CR, Foekens JA, Zhang Y, Wang Y, Marcom PK, Marks JR, Febbo PG, Nevins JR, Potti A, Blackwell KL (2008) Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol 26(20):3324–3330. https://doi.org/10.1200/JCO.2007.14.2471
Aalders KC, Postma EL, Strobbe LJ, Der Heiden-Van V, Der Loo M, Sonke GS, Boersma LJ, Van Diest PJ, Siesling S, Van Dalen T (2016) Contemporary locoregional recurrence rates in young patients with early-stage breast cancer. J Clin Oncol 34(18):2107–2114. https://doi.org/10.1200/JCO.2015.64.3536
Cao JQ, Truong PT, Olivotto IA, Olson R, Coulombe G, Keyes M, Weir L, Gelmon K, Bernstein V, Woods R, Speers C, Tyldesley S (2014) Should women younger than 40 years of age with invasive breast cancer have a mastectomy? 15-year outcomes in a population-based cohort. Int J Radiat Oncol Biol Phys 90(3):509–517. https://doi.org/10.1016/j.ijrobp.2014.06.041
Ye JC, Yan W, Christos PJ, Nori D, Ravi A (2015) Equivalent survival with mastectomy or breast-conserving surgery plus radiation in young women aged < 40 years with early-stage breast cancer: a national registry-based stage-by-stage comparison. Clin Breast Cancer 15(5):390–397. https://doi.org/10.1016/j.clbc.2015.03.012
Azim HA Jr, Partridge AH (2014) Biology of breast cancer in young women. Breast Cancer Res 16(4):427. https://doi.org/10.1186/s13058-014-0427-5
Collins LC, Marotti JD, Gelber S, Cole K, Ruddy K, Kereakoglow S, Brachtel EF, Schapira L, Come SE, Winer EP, Partridge AH (2012) Pathologic features and molecular phenotype by patient age in a large cohort of young women with breast cancer. Breast Cancer Res Treat 131(3):1061–1066. https://doi.org/10.1007/s10549-011-1872-9
Lund MJ, Butler EN, Hair BY, Ward KC, Andrews JH, Oprea-Ilies G, Bayakly AR, O'Regan RM, Vertino PM, Eley JW (2010) Age/race differences in HER2 testing and in incidence rates for breast cancer triple subtypes: a population-based study and first report. Cancer 116(11):2549–2559. https://doi.org/10.1002/cncr.25016
Rossi L, Mazzara C, Pagani O (2019) Diagnosis and treatment of breast cancer in young women. Curr Treat Options Oncol 20(12):86. https://doi.org/10.1007/s11864-019-0685-7
Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, Weaver DL, Winchester DJ, Hortobagyi GN (2017) Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA 67(4):290–303. https://doi.org/10.3322/caac.21393
Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark G, Edge SB, Hayes DF, Hughes LL, Hutter RV, Morrow M, Page DL, Recht A, Theriault RL, Thor A, Weaver DL, Wieand HS, Greene FL (2002) Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol 20(17):3628–3636. https://doi.org/10.1200/jco.2002.02.026
Woodward WA, Vinh-Hung V, Ueno NT, Cheng YC, Royce M, Tai P, Vlastos G, Wallace AM, Hortobagyi GN, Nieto Y (2006) Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol 24(18):2910–2916. https://doi.org/10.1200/jco.2005.03.1526
Vinh-Hung V, Verkooijen HM, Fioretta G, Neyroud-Caspar I, Rapiti E, Vlastos G, Deglise C, Usel M, Lutz JM, Bouchardy C (2009) Lymph node ratio as an alternative to pN staging in node-positive breast cancer. J Clin Oncol 27(7):1062–1068. https://doi.org/10.1200/jco.2008.18.6965
Chagpar AB, Camp RL, Rimm DL (2011) Lymph node ratio should be considered for incorporation into staging for breast cancer. Ann Surg Oncol 18(11):3143–3148. https://doi.org/10.1245/s10434-011-2012-9
Hatoum HA, Jamali FR, El-Saghir NS, Musallam KM, Seoud M, Dimassi H, Abbas J, Khalife M, Boulos FI, Tawil AN, Geara FB, Salem Z, Shamseddine AA, Al-Feghali K, Shamseddine AI (2009) Ratio between positive lymph nodes and total excised axillary lymph nodes as an independent prognostic factor for overall survival in patients with nonmetastatic lymph node-positive breast cancer. Ann Surg Oncol 16(12):3388–3395. https://doi.org/10.1245/s10434-009-0653-8
Vinh-Hung V, Verschraegen C, Promish DI, Cserni G, Van de Steene J, Tai P, Vlastos G, Voordeckers M, Storme G, Royce M (2004) Ratios of involved nodes in early breast cancer. Breast Cancer Res 6(6):R680–688. https://doi.org/10.1186/bcr934
Chen S, Liu Y, Huang L, Chen CM, Wu J, Shao ZM (2014) Lymph node counts and ratio in axillary dissections following neoadjuvant chemotherapy for breast cancer: a better alternative to traditional pN staging. Ann Surg Oncol 21(1):42–50. https://doi.org/10.1245/s10434-013-3245-6
Cho DH, Bae SY, You JY, Kim HK, Chang YW, Choi YJ, Woo SU, Son GS, Lee JB, Bae JW, Jung SP (2018) Lymph node ratio as an alternative to pN staging for predicting prognosis after neoadjuvant chemotherapy in breast cancer. Kaohsiung J Med Sci 34(6):341–347. https://doi.org/10.1016/j.kjms.2017.12.015
Danko ME, Bennett KM, Zhai J, Marks JR, Olson JA, Jr. (2010) Improved staging in node-positive breast cancer patients using lymph node ratio: results in 1,788 patients with long-term follow-up. Journal of the American College of Surgeons 210 (5):797–805 e791, 805–797. https://doi.org/10.1016/j.jamcollsurg.2010.02.045
Truong PT, Berthelet E, Lee J, Kader HA, Olivotto IA (2005) The prognostic significance of the percentage of positive/dissected axillary lymph nodes in breast cancer recurrence and survival in patients with one to three positive axillary lymph nodes. Cancer 103(10):2006–2014. https://doi.org/10.1002/cncr.20969
Kim J, Park W, Kim JH, Choi DH, Kim YJ, Lee ES, Shin KH, Kim JH, Kim K, Kim YB, Ahn SJ, Lee JH, Chun M, Lee HS, Kim JS, Cha J (2019) Clinical significance of lymph-node ratio in determining supraclavicular lymph-node radiation therapy in pn1 breast cancer patients who received breast-conserving treatment (KROG 14–18): a multicenter study. Cancers. https://doi.org/10.3390/cancers11050680
Agarwal R, Philip A, Pavithran K, Rajanbabu A, Goel G, Vijaykumar DK (2019) Prognostic significance of residual nodal burden using lymph node ratio in locally advanced breast cancer after neoadjuvant chemotherapy. Indian J Cancer 56(3):228–235. https://doi.org/10.4103/ijc.IJC_652_18
Camp LR (2004) X-Tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10(21):7252–7259
Kudela E, Samec M, Kubatka P, Nachajova M, Laucekova Z, Liskova A, Dokus K, Biringer K, Simova D, Gabonova E, Dankova Z, Biskupska Bodova K, Zubor P, Trog D (2019) Breast cancer in young women: status quo and advanced disease management by a predictive, preventive, and personalized approach. Cancers. https://doi.org/10.3390/cancers11111791
Keramatinia A, Mousavi-Jarrahi SH, Hiteh M, Mosavi-Jarrahi A (2014) Trends in incidence of breast cancer among women under 40 in Asia. Asian Pac J Cancer Prev 15(3):1387–1390
Ravdin PM, Siminoff LA, Davis GJ, Mercer MB, Hewlett J, Gerson N, Parker HL (2001) Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol 19(4):980–991. https://doi.org/10.1200/jco.2001.19.4.980
Haybittle JL, Blamey RW, Elston CW, Johnson J, Doyle PJ, Campbell FC, Nicholson RI, Griffiths K (1982) A prognostic index in primary breast cancer. Br J Cancer 45(3):361–366. https://doi.org/10.1038/bjc.1982.62
Galea MH, Blamey RW, Elston CE, Ellis IO (1992) The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 22(3):207–219. https://doi.org/10.1007/bf01840834
Wishart GC, Azzato EM, Greenberg DC, Rashbass J, Kearins O, Lawrence G, Caldas C, Pharoah PD (2010) PREDICT: a new UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 12(1):R1. https://doi.org/10.1186/bcr2464
Engelhardt EG, Garvelink MM, de Haes JH, van der Hoeven JJ, Smets EM, Pieterse AH, Stiggelbout AM (2014) Predicting and communicating the risk of recurrence and death in women with early-stage breast cancer: a systematic review of risk prediction models. J Clin Oncol 32(3):238–250. https://doi.org/10.1200/jco.2013.50.3417
Lambertini M, Pinto AC, Ameye L, Jongen L, Del Mastro L, Puglisi F, Poggio F, Bonotto M, Floris G, Van Asten K, Wildiers H, Neven P, de Azambuja E, Paesmans M, Azim HA Jr (2016) The prognostic performance of Adjuvant! Online and Nottingham Prognostic Index in young breast cancer patients. Br J Cancer 115(12):1471–1478. https://doi.org/10.1038/bjc.2016.359
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K (2010) Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 252(3):426–432. https://doi.org/10.1097/SLA.0b013e3181f08f32
Giuliano AE, Hunt KK, Ballman KV, Beitsch PD, Whitworth PW, Blumencranz PW, Leitch AM, Saha S, McCall LM, Morrow M (2011) Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305(6):569–575. https://doi.org/10.1001/jama.2011.90
Galimberti V, Cole BF, Zurrida S, Viale G, Luini A, Veronesi P, Baratella P, Chifu C, Sargenti M, Intra M, Gentilini O, Mastropasqua MG, Mazzarol G, Massarut S, Garbay JR, Zgajnar J, Galatius H, Recalcati A, Littlejohn D, Bamert M, Colleoni M, Price KN, Regan MM, Goldhirsch A, Coates AS, Gelber RD, Veronesi U (2013) Axillary dissection versus no axillary dissection in patients with sentinel-node micrometastases (IBCSG 23–01): a phase 3 randomised controlled trial. Lancet Oncol 14(4):297–305. https://doi.org/10.1016/s1470-2045(13)70035-4
Galimberti V, Cole BF, Viale G, Veronesi P, Vicini E, Intra M, Mazzarol G, Massarut S, Zgajnar J, Taffurelli M, Littlejohn D, Knauer M, Tondini C, Di Leo A, Colleoni M, Regan MM, Coates AS, Gelber RD, Goldhirsch A (2018) Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23–01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol 19(10):1385–1393. https://doi.org/10.1016/s1470-2045(18)30380-2
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, MacKinnon E, McGale P, McHugh T, Peto R, Taylor C, Wang Y (2005) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366(9503):2087–2106. https://doi.org/10.1016/s0140-6736(05)67887-7
Weir L, Speers C, D'Yachkova Y, Olivotto IA (2002) Prognostic significance of the number of axillary lymph nodes removed in patients with node-negative breast cancer. J Clin Oncol 20(7):1793–1799. https://doi.org/10.1200/jco.2002.07.112
Axelsson CK, Mouridsen HT, Zedeler K (1992) Axillary dissection of level I and II lymph nodes is important in breast cancer. The Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer 28a(8–9):1415–1418. https://doi.org/10.1016/0959-8049(92)90534-9
Vinh-Hung V, Joseph SA, Coutty N, Ly BH, Vlastos G, Nguyen NP (2010) Age and axillary lymph node ratio in postmenopausal women with T1–T2 node positive breast cancer. Oncologist 15(10):1050–1062. https://doi.org/10.1634/theoncologist.2010-0044
Qin T, Zeng YD, Lu Q, Zhang X, Qin GE, Zheng Q, Xu F, Peng R, Yuan Z, Wang S (2017) Nomogram model of LNR predicts survival in premenopausal patients with node-positive luminal breast cancer. Anticancer Res 37(8):4575–4586. https://doi.org/10.21873/anticanres.11856
Truong PT, Lesperance M, Li KH, MacFarlane R, Speers CH, Chia S (2010) Micrometastatic node-positive breast cancer: long-term outcomes and identification of high-risk subsets in a large population-based series. Ann Surg Oncol 17(8):2138–2146. https://doi.org/10.1245/s10434-010-0954-y
Engelhardt EG, van den Broek AJ, Linn SC, Wishart GC, Rutgers EJT, van de Velde AO, Smit V, Voogd AC, Siesling S, Brinkhuis M, Seynaeve C, Westenend PJ, Stiggelbout AM, Tollenaar R, van Leeuwen FE, van Veer LJ, Ravdin PM, Pharaoh PDP, Schmidt MK (2017) Accuracy of the online prognostication tools PREDICT and Adjuvant! for early-stage breast cancer patients younger than 50 years. Eur J Cancer 78:37–44. https://doi.org/10.1016/j.ejca.2017.03.015
Maggard MA, O'Connell JB, Lane KE, Liu JH, Etzioni DA, Ko CY (2003) Do young breast cancer patients have worse outcomes? J Surg Res 113(1):109–113. https://doi.org/10.1016/s0022-4804(03)00179-3
Gong Y, Ji P, Sun W, Jiang YZ, Hu X, Shao ZM (2018) Development and validation of nomograms for predicting overall and breast cancer-specific survival in young women with breast cancer: a population-based study. Transl Oncol 11(6):1334–1342. https://doi.org/10.1016/j.tranon.2018.08.008
Agrup M, Stal O, Olsen K, Wingren S (2000) C-erbB-2 overexpression and survival in early onset breast cancer. Breast Cancer Res Treat 63(1):23–29. https://doi.org/10.1023/a:1006498721508
Funding
This study was supported by grants from the National Natural Science Foundation of China (81472731, 81972467), the Guangdong Natural Science Foundation (2020A1515010458), and the Guangdong Science and Technology Department (2017B030314026).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2020_5870_MOESM1_ESM.tif
ESM_1: Receiver operating characteristic curves comparing the use of the nomograms and AJCC TNM staging system to predict the 3-years OS (A), 5-years overall OS (B), and the 3-years BCSS (C), the 5-years BCSS (D) for young patients with breast cancer in the training group. (OS, overall survival; BCSS, breast cancer-specific survival; AUC, area under the curve) (TIF 16678 kb)
10549_2020_5870_MOESM2_ESM.tif
ESM_2: Decision curve analysis of the nomograms and AJCC TNM staging system for predicting the 3-years OS (A), 5-years overall OS (B), and the 3-years BCSS (C), the 5-years BCSS (D) for young patients with breast cancer in the training group. (OS, overall survival; BCSS, breast cancer-specific survival) (TIF 15724 kb)
Rights and permissions
About this article
Cite this article
Sun, Y., Li, Y., Wu, J. et al. Nomograms for prediction of overall and cancer-specific survival in young breast cancer. Breast Cancer Res Treat 184, 597–613 (2020). https://doi.org/10.1007/s10549-020-05870-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05870-5