Abstract
Purpose
Combinations of endocrine therapy (ET) and targeted therapy (CDK4/6 or mTOR inhibitors) are standard of care for HR+/HER2− metastatic breast cancer (MBC). When ET is not effective, chemotherapy is commonly used. However, clinical outcomes of chemotherapy in the endocrine-resistant setting are limited. The purpose of this study was to identify predictive factors and the compare efficacies of chemotherapy agents in endocrine-resistant MBC.
Methods
We conducted a retrospective study of patients with HR+/HER2− MBC who received chemotherapy after progression on ET with or without targeted therapy at MD Anderson Cancer Center from 1999 to 2017. We collected baseline clinicopathological and all treatment data. Primary endpoint was time to treatment failure (TTF) of first-line chemotherapy for MBC.
Results
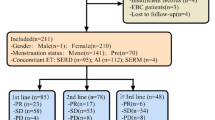
For the 1258 patients analyzed, mean age was 55.3 years (range 21–91). Previous treatment with targeted therapy was recorded for 390 patients (31%): 264 with CDK4/6 inhibitor, 205 with mTOR inhibitor, and 79 treated with both. The most frequent chemotherapy agents were capecitabine (48.9%) and taxanes (28.6%). After adjustment for all factors in a multivariate model, previous treatment with a CDK4/6 inhibitor had the strongest negative effect on TTF regardless of ET duration (hazard ratio [HR] 1.84; 95%CI 1.49–2.27; p < 0.001). Conversely, capecitabine had significantly longer median TTF than taxanes regardless of whether patients had prior exposure to taxanes in primary setting (6.1 vs 4.9 months; HR 0.64; 95%CI 0.55–0.75; p < 0.001).
Conclusions
Previous exposure to CDK4/6 inhibitor had a negative predictive effect for the efficacy of chemotherapy. Capecitabine had the best efficacy against endocrine-resistant breast cancer.



Similar content being viewed by others
Abbreviations
- ET:
-
Endocrine therapy
- HR+:
-
Hormone receptor-positive
- HER2−:
-
Human epidermal growth factor receptor 2-negative
- CDK4/6:
-
Cyclin-dependent kinase 4 and 6
- mTOR:
-
Mammalian target of rapamycin
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- IBC:
-
Inflammatory breast cancer
References
National Comprehensive Cancer Network (2020) NCCN clinical practice guidelines in oncology: breast cancer (version 3.2019). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 3 Jan 2020
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, Andre F, Harbeck N, Aguilar Lopez B, Barrios CH, Bergh J, Biganzoli L, Boers-Doets CB, Cardoso MJ, Carey LA, Cortes J, Curigliano G, Dieras V, El Saghir NS, Eniu A, Fallowfield L, Francis PA, Gelmon K, Johnston SRD, Kaufman B, Koppikar S, Krop IE, Mayer M, Nakigudde G, Offersen BV, Ohno S, Pagani O, Paluch-Shimon S, Penault-Llorca F, Prat A, Rugo HS, Sledge GW, Spence D, Thomssen C, Vorobiof DA, Xu B, Norton L, Winer EP (2018) 4th ESO-ESMO International consensus guidelines for advanced breast cancer (ABC 4). Ann Oncol 29(8):1634–1657. https://doi.org/10.1093/annonc/mdy192
Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN, Fallowfield L, Fowble B, Ingle JN, Jahanzeb M, Johnston SR, Korde LA, Khatcheressian JL, Mehta RS, Muss HB, Burstein HJ (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 34(25):3069–3103. https://doi.org/10.1200/JCO.2016.67.1487
Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, Chow L, Sohn J, Lee KS, Campos-Gomez S, Villanueva-Vazquez R, Jung KH, Chakravartty A, Hughes G, Gounaris I, Rodriguez-Lorenc K, Taran T, Hurvitz S, Tripathy D (2019) Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 381(4):307–316. https://doi.org/10.1056/NEJMoa1903765
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke E-M, Conte P, Lu Y, Barriga S, Hurt K, Frenzel M, Johnston S, Llombart-Cussac A (2019) The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor–positive, erbb2-negative breast cancer that progressed on endocrine therapy MONARCH 2: a randomized clinical trial. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.4782
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. https://doi.org/10.1056/NEJMoa1109653
Sledge GW Jr, Toi M, Neven P, Sohn J, Inoue K, Pivot X, Burdaeva O, Okera M, Masuda N, Kaufman PA, Koh H, Grischke EM, Frenzel M, Lin Y, Barriga S, Smith IC, Bourayou N, Llombart-Cussac A (2017) MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 35(25):2875–2884. https://doi.org/10.1200/JCO.2017.73.7585
Prat A, Fan C, Fernandez A, Hoadley KA, Martinello R, Vidal M, Viladot M, Pineda E, Arance A, Munoz M, Pare L, Cheang MC, Adamo B, Perou CM (2015) Response and survival of breast cancer intrinsic subtypes following multi-agent neoadjuvant chemotherapy. BMC Med 13:303. https://doi.org/10.1186/s12916-015-0540-z
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E (2012) Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer 48(18):3342–3354. https://doi.org/10.1016/j.ejca.2012.05.023
Zhu Y, Liu Y, Zhang C, Chu J, Wu Y, Li Y, Liu J, Li Q, Li S, Shi Q, Jin L, Zhao J, Yin D, Efroni S, Su F, Yao H, Song E, Liu Q (2018) Tamoxifen-resistant breast cancer cells are resistant to DNA-damaging chemotherapy because of upregulated BARD1 and BRCA1. Nat Commun 9(1):1595. https://doi.org/10.1038/s41467-018-03951-0
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC, American Society of Clinical Oncology, Oncology College of American Pathologists (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 134(7):e48–e72. https://doi.org/10.1043/1543-2165-134.7.e48
Harbeck N, Gnant M (2017) Breast cancer. Lancet (London, England) 389(10074):1134–1150. https://doi.org/10.1016/s0140-6736(16)31891-8
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Andre F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M (2018) Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 379(20):1926–1936. https://doi.org/10.1056/NEJMoa1810527
Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Zhang K, Theall KP, Jiang Y, Bartlett CH, Koehler M, Slamon D (2016) Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol 17(4):425–439. https://doi.org/10.1016/S1470-2045(15)00613-0
Stockler MR, Harvey VJ, Francis PA, Byrne MJ, Ackland SP, Fitzharris B, Van Hazel G, Wilcken NR, Grimison PS, Nowak AK, Gainford MC, Fong A, Paksec L, Sourjina T, Zannino D, Gebski V, Simes RJ, Forbes JF, Coates AS (2011) Capecitabine versus classical cyclophosphamide, methotrexate, and fluorouracil as first-line chemotherapy for advanced breast cancer. J Clin Oncol 29(34):4498–4504. https://doi.org/10.1200/JCO.2010.33.9101
Harbeck N, Saupe S, Jager E, Schmidt M, Kreienberg R, Muller L, Otremba BJ, Waldenmaier D, Dorn J, Warm M, Scholz M, Untch M, de Wit M, Barinoff J, Luck HJ, Harter P, Augustin D, Harnett P, Beckmann MW, Al-Batran SE, Investigators P (2017) A randomized phase III study evaluating pegylated liposomal doxorubicin versus capecitabine as first-line therapy for metastatic breast cancer: results of the PELICAN study. Breast Cancer Res Treat 161(1):63–72. https://doi.org/10.1007/s10549-016-4033-3
Talbot DC, Moiseyenko V, Van Belle S, O'Reilly SM, Alba Conejo E, Ackland S, Eisenberg P, Melnychuk D, Pienkowski T, Burger HU, Laws S, Osterwalder B (2002) Randomised, phase II trial comparing oral capecitabine (Xeloda) with paclitaxel in patients with metastatic/advanced breast cancer pretreated with anthracyclines. Br J Cancer 86(9):1367–1372. https://doi.org/10.1038/sj.bjc.6600261
Oshaughnessy JA, Blum J, Moiseyenko V, Jones SE, Miles D, Bell D, Rosso R, Mauriac L, Osterwalder B, Burger HU, Laws S (2001) Randomized, open-label, phase II trial of oral capecitabine (Xeloda) vs. a reference arm of intravenous CMF (cyclophosphamide, methotrexate and 5-fluorouracil) as first-line therapy for advanced/metastatic breast cancer. Ann Oncol 12(9):1247–1254. https://doi.org/10.1023/a:1012281104865
Pazdur R (2008) Endpoints for assessing drug activity in clinical trials. Oncologist 13(Suppl 2):19–21. https://doi.org/10.1634/theoncologist.13-S2-19
Acknowledgements
We would like to thank Sunita Patterson (Scientific Publications, Research Medical Library, MD Anderson Cancer Center) for her help in editing this article and Limin Hsu, Modesto Patangan, and Akshara Raghavendra, our data management team, for help with our database.
Funding
This research was supported by the Morgan Welch Inflammatory Breast Cancer Research Program, a State of Texas Rare and Aggressive Breast Cancer Research Program Grant, and National Institutes of Health/National Cancer Institute grant P30 CA016672 (Cancer Center Support Grant; used the Biostatistics Resource Group and the Clinical and Translational Research Center).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of The University of Texas MD Anderson Cancer Center (PA18-1135) approved this study.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chainitikun, S., Long, J.P., Rodriguez-Bautista, R. et al. The efficacy of first-line chemotherapy in endocrine-resistant hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) metastatic breast cancer. Breast Cancer Res Treat 183, 729–739 (2020). https://doi.org/10.1007/s10549-020-05837-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05837-6