Abstract
Purpose
In Brazil, the available cancer registries are deficient in number and quality and, hence, little information is known regarding sociodemographic, clinicopathological characteristics, treatment patterns, and outcomes of breast cancer (BC) patients. We performed the AMAZONA III/ GBECAM 0115 study and in this analysis, we describe patients’ characteristics at diagnosis and their association with health insurance type.
Methods
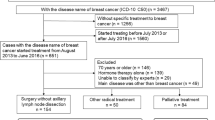
This is a prospective cohort study developed in 23 sites in Brazil including women with newly diagnosed invasive BC from January 2016 to March 2018. In order to compare healthcare insurance type, we considered patients who were treated under the Brazilian public health system as publicly insured, and women who had private insurance or paid for their treatment as privately insured.
Results
A total of 2950 patients were included in the study. Median age at diagnosis was 53.9 years; 63.1% were publicly insured. The majority of patients (68.6%) had stage II–III breast cancer and ductal carcinoma histology (80.9%). The most common breast cancer subtype was luminal A-like (48.0%) followed by luminal B-HER2 positive-like (17.0%) and triple-negative (15.6%). Luminal A was more frequent in private (53.7% vs. 44.2%, p < .0001) than public, whereas Luminal B HER2-positive (19.2% vs. 14.2%, p = 0.0012) and HER2-positive (8.8% vs. 5.1%, p = 0.0009) were more common in patients with public health system coverage. Only 34% of patients were diagnosed by screening exams. Privately insured patients were more frequently diagnosed with stage I disease when compared to publicly insured patients; publicly insured patients had more stage III (33.5% vs. 14.7%; p-value < 0.0001) disease than privately insured ones. Breast cancer was detected by symptoms more frequently in publicly than in privately insured patients (74.2% vs 25.8%, respectively; p-value < 0.0001).
Conclusions
Patients with public health coverage were diagnosed with symptomatic disease, later stages and more aggressive subtypes when compared to privately insured patients.



Similar content being viewed by others
Abbreviations
- BC:
-
Breast cancer
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- LMIC:
-
Low to middle-income countries
- PR:
-
Progesterone receptor
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Instituto Nacional de Câncer José Alencar Gomes (2018) Estimativa 2018: Incidência de Câncer no Brasil. INCA, Rio de Janeiro
Bray F, McCarron P, Parkin DM (2004) The changing global patterns of female breast cancer incidence and mortality. Breast Cancer Res 6:229
Goss PE, Lee BL, Badovinac-Crnjevic T et al (2013) Planning cancer control in Latin America and the Caribbean. Lancet Oncol 14:391–436. https://doi.org/10.1016/S1470-2045(13)70048-2
Simon SD, Bines J, Werutsky G et al (2019) Characteristics and prognosis of stage I-III breast cancer subtypes in Brazil: the AMAZONA retrospective cohort study. Breast 44:113–119
Liedke PER, Finkelstein DM, Szymonifka J et al (2014) Outcomes of breast cancer in brazil related to health care coverage: a retrospective cohort study. Cancer Epidemiol Prev Biomark 23:126–133
Serrano-Gomez SJ, Fejerman L, Zabaleta J (2018) Breast cancer in latinas: a focus on intrinsic subtypes distribution. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol 27:3–10
Yedjou CG, Sims JN, Miele L et al (2019) Health and racial disparity in breast cancer. Adv Exp Med Biol 1152:31–49
Palmer JR, Boggs DA, Wise LA, Ambrosone CB, Adams-Campbell LL, Rosenberg L (2011) Parity and lactation in relation to estrogen receptor negative breast cancer in African American women. Cancer Epidemiol Biomark Prev 20(9):1883–1891
Hall IJ, Moorman PG, Millikan RC, Newman B (2005) Comparative analysis of breast cancer risk factors among African-American women and White women. Am J Epidemiol 161(1):40–51
Chlebowski RT, Chen Z, Anderson GL et al (2005) Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J Natl Cancer Inst 97(6):439–448
Pinheiro SP, Holmes MD, Pollak MN, Barbieri RL, Hankinson SE (2005) Racial differences in premenopausal endogenous hormones. Cancer Epidemiol Biomark Prev 14(9):2147–2153
Porter PL, Lund MJ, Lin MG et al (2004) Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer 100(12):2533–2542
Martin DN, Boersma BJ, Yi M et al (2009) Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PLoS ONE 4(2):e4531
Gukas ID, Girling AC, Mandong BM, Prime W, Jennings BA, Leinster SJ (2008) A comparison of clinicopathological features and molecular markers in british and nigerian women with breast cancer. Clin Med Oncol 2:347–351
Loo LW, Wang Y, Flynn EM et al (2011) Genome-wide copy number alterations in subtypes of invasive breast cancers in young white and African American women. Breast Cancer Res Treat 127(1):297–308
Ray M, Polite BN (2010) Triple-negative breast cancers: a view from 10,000 feet. Cancer J 16(1):17–22
Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE (2009) Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol 169(10):1251–1259
Dunnwald LK, Rossing MA, Li CI (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9(1):R6
Franco-Marina F, López-Carrillo L, Keating NL, Arreola-Ornelas H, Marie KF (2015) Breast cancer age at diagnosis patterns in four Latin American Populations: A comparison with North American countries. Cancer Epidemiol 39(6):831–837
Ginsburg O, Bray F, Coleman MP et al (2017) The global burden of women's cancers: a grand challenge in global health. Lancet 389(10071):847–860
Silva PRV, Carvalho DBF, Trajano V et al (2017) Using google trends data to study public interest in breast cancer screening in Brazil: why not a pink february? JMIR Public Health Surveill. https://doi.org/10.2196/publichealth.7015
Ministério da Saúde Mamografias feitas no País crescem 37% em seis anos (2017). In: Gov. Bras. https://www.brasil.gov.br/noticias/saude/2016/10/mamografias-feitas-no-pais-crescem-37-em-seis-anos. Accessed 26 Feb 2019
dos Figueiredo FWS, do Almeida TCC, Cardial DT et al (2017) The role of health policy in the burden of breast cancer in Brazil. BMC Womens Health 17:121
Dados Gerais—ANS—Agência Nacional de Saúde Suplementar. https://www.ans.gov.br/perfil-do-setor/dados-gerais. Accessed 20 Mar 2019
Gonzaga CMR, Freitas-Junior R, Curado M-P et al (2015) Temporal trends in female breast cancer mortality in Brazil and correlations with social inequalities: ecological time-series study. BMC Public Health 15:96
Acknowledgements
We would like to thank the patients, the research teams, and investigators for the dedication to this study. We acknowledge Instituto Avon, Itaú Seguros, and Grupo Bamaq for the financial support under the PRONON (Ministry of Health, Brazil) program and SAS Institute Inc. for providing license-free access to SAS®.
Funding
This study was funded by a Grant from Programa Nacional de Apoio à Atenção Oncológica (PRONON) of the Brazilian Health Ministry (No. 25000.173.901/2013–73).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
DDR is a consultant for: Roche, Novartis, AstraZeneca, Lilly, GSK, Sanofi, Libbs, Eisai, Pfizer, MSD, Dr Reddy’s. Research funding is obtained from Amgen, Roche, GSK, L’Òreal. Expert testimony for: Roche, Novartis, Pfizer, AstraZeneca, Lilly, MSD. Travel, Accommodations, Expenses: Roche, Novartis, Lilly, Amgen. HR: speaker honorarium from Company Libbs Farmaceutica. CHB: Stock and Other Ownership Interests: Biomarker, MedSIR, Tummi Honoraria: Novartis, Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai. Consulting or Advisory Role: Boehringer Ingelheim, Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical. Research Funding: Pfizer, Novartis, Amgen, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Genentech, Eli Lilly, Sanofi, Taiho Pharmaceutical, Mylan, Merrimack, Merck, AbbVie, Astellas Pharma, Biomarin, Bristol-Myers Squibb, Daiichi Sankyo, Abraxis BioScience, AB Science, Asana Biosciences, Medivation, Exelixis, ImClone Systems, LEO Pharma, Millennium, Janssen, Atlantis Clinica, INC Research, Halozyme, Covance, Celgene, inVentiv Health. Travel, Accommodations, Expenses: Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology. TR received research funding from AstraZeneca and speaker honoraria from AstraZeneca, Lilly, Novartis, Pfizer and PierreFabre. CSF: Advisory Board Chair—Clinica AMO, Advisory Board Member—Indivumed (based in Hamburg-Germany), Multiple affiliations: ASCO, SBOC. MSM: Honoraria for speaker and advisory board engagements: Roche, Novartis, Astrazeneca, Pfizer, Eisai, Genomic Health, Lilly, Oncologia Brasil, Amgen, DASA/GeneOne, Educational support (Roche, Pfizer), Principal and sub investigator of clinical trials from Novartis, Roche, Lilly, Pfizer. Stock Ownership: Biotoscana, Hypera, Fleury. JB, GW, EC, GSQ, VCCL, RFJ, JOC, KE, SC, BVE, YN, VD, NL, RCC, DAPA, CM, GZV, GB, AM, MC, FZ, RJG, and SDS declare that they have no conflicts of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All study subjects provided written informed consent before entering the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosa, D.D., Bines, J., Werutsky, G. et al. The impact of sociodemographic factors and health insurance coverage in the diagnosis and clinicopathological characteristics of breast cancer in Brazil: AMAZONA III study (GBECAM 0115). Breast Cancer Res Treat 183, 749–757 (2020). https://doi.org/10.1007/s10549-020-05831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05831-y