Abstract
Purpose
To examine (1) the trend and associated factors of Oncotype DX (ODX) use among hormone receptor-positive (HR+) breast cancer (BC) patients in 2004–2015; (2) the trend of reported chemotherapy by Recurrence Score (RS); and (3) the survival differences associated with ODX use.
Methods
ODX data from Genomic Health Inc. were linked with 17 SEER registries data. HR + BC cases with lymph node negative (N0) or 1–3 positive LNs (N1) from 2004–2015 were analyzed. The Cochrane-Armitage trend test, logistic regression, Kaplan–Meier survival curve, and stratified Cox model were performed. Survival analysis was restricted to HR+/HER2− patients from 2010 to 2014, matched on propensity score.
Results
ODX use increased substantially from 2004 to 2015 (N0: 2.0% to 42.7%; N1: 0.3% to 27.9%). Non-Hispanic black and Medicaid insured patients had lower odds of receiving ODX. N0 patients with moderately differentiated or 2.1–5.0 cm tumor and N1 patients with well-differentiated or < 2.0 cm tumor had higher odds of using ODX. The reported chemotherapy use decreased significantly with low and intermediate RS, and increased for high RS among N0 patients. ODX use was associated with better breast cancer-specific survival [hazard ratio (95% CI) N0 1.96 (1.60–2.41), N1 1.90 (1.42–2.54)] and overall survival [N0 2.06 (1.83–2.31), N1 1.72 (1.42–2.09)], especially in the first 36 months.
Conclusion
ODX use has increased significantly since 2004, nonetheless disparities remain, especially for racial/ethnic minorities and Medicaid insured patients. Administering chemotherapy based on ODX results has been improved among N0 patients. Patients receiving ODX had better survival than those not.



Similar content being viewed by others
References
Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351(27):2817–2826. https://doi.org/10.1056/NEJMoa041588
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, Zujewski J, Lively T, Badve SS, Saphner TJ, Wagner LI, Whelan TJ, Ellis MJ, Paik S, Wood WC, Ravdin P, Keane MM, Gomez Moreno HL, Reddy PS, Goggins TF, Mayer IA, Brufsky AM, Toppmeyer DL, Kaklamani VG, Atkins JN, Berenberg JL, Sledge GW (2015) Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med 373(21):2005–2014. https://doi.org/10.1056/NEJMoa1510764
Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N (2006) Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 24(23):3726–3734. https://doi.org/10.1200/jco.2005.04.7985
NCCN Clinical Practice Guideline in Oncology (NCCN Guideline®) for Breast Cancer. © National Comprehensive Cancer Network, Inc.
Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL, Davidson NE, Sledge GW, Winer EP, Hudis C, Ingle JN, Perez EA, Pritchard KI, Shepherd L, Gralow JR, Yoshizawa C, Allred DC, Osborne CK, Hayes DF (2010) Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 11(1):55–65. https://doi.org/10.1016/s1470-2045(09)70314-6
Dowsett M, Cuzick J, Wale C, Forbes J, Mallon EA, Salter J, Quinn E, Dunbier A, Baum M, Buzdar A, Howell A, Bugarini R, Baehner FL, Shak S (2010) Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol 28(11):1829–1834. https://doi.org/10.1200/jco.2009.24.4798
Hassett MJ, Silver SM, Hughes ME, Blayney DW, Edge SB, Herman JG, Hudis CA, Marcom PK, Pettinga JE, Share D, Theriault R, Wong YN, Vandergrift JL, Niland JC, Weeks JC (2012) Adoption of gene expression profile testing and association with use of chemotherapy among women with breast cancer. J Clin Oncol 30(18):2218–2226. https://doi.org/10.1200/jco.2011.38.5740
Dinan MA, Mi X, Reed SD, Hirsch BR, Lyman GH, Curtis LH (2015) Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the medicare population, 2005–2009. JAMA Oncol 1(2):158–166. https://doi.org/10.1001/jamaoncol.2015.43
Cress RD, Chen YS, Morris CR, Chew H, Kizer KW (2016) Underutilization of gene expression profiling for early-stage breast cancer in California. Cancer Causes Control 27(6):721–727. https://doi.org/10.1007/s10552-016-0743-4
Peethambaram PP, Hoskin TL, Day CN, Goetz MP, Habermann EB, Boughey JC (2017) Use of 21-gene recurrence score assay to individualize adjuvant chemotherapy recommendations in ER+/HER2- node positive breast cancer—a National Cancer Database study. NPJ Breast Cancer 3:41. https://doi.org/10.1038/s41523-017-0044-4
Surveillance, Epidemiology, and End Results Program (2019) https://seer.cancer.gov/about/overview.html. Accessed 15 Oct 2019
Petkov VI, Miller DP, Howlader N, Gliner N, Howe W, Schussler N, Cronin K, Baehner FL, Cress R, Deapen D, Glaser SL, Hernandez BY, Lynch CF, Mueller L, Schwartz AG, Schwartz SM, Stroup A, Sweeney C, Tucker TC, Ward KC, Wiggins C, Wu XC, Penberthy L, Shak S (2016) Breast-cancer-specific mortality in patients treated based on the 21-gene assay: a SEER population-based study. NPJ Breast Cancer 2:16017. https://doi.org/10.1038/npjbcancer.2016.17
National Cancer Institute. Surveillance, Epidemiology, and End Results Program (2019) https://seer.cancer.gov/seerstat/variables/seer/insurance-recode/. Accessed 15 Oct 2019
Agresti A (2002) Categorical dta analysis, 2ed. Wiley, New York
Coca-Perraillon M (2019) Local and global optimal propensity score matching. https://support.sas.com/resources/papers/proceedings/proceedings/forum2007/185-2007.pdf. Accessed 15 Oct 2019
Kleinbaum DG, Klein M (2012) Survival analysis, 3rd edn. Springer, New York
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC Jr (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312. https://doi.org/10.1200/jco.2007.14.2364
Harris LN, Ismaila N, McShane LM, Andre F, Collyar DE, Gonzalez-Angulo AM, Hammond EH, Kuderer NM, Liu MC, Mennel RG, Van Poznak C, Bast RC, Hayes DF (2016) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol 34(10):1134–1150. https://doi.org/10.1200/jco.2015.65.2289
Enewold L, Geiger AM, Zujewski J, Harlan LC (2015) Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat 151(1):149–156. https://doi.org/10.1007/s10549-015-3366-7
Press DJ, Ibraheem A, Dolan ME, Goss KH, Conzen S, Huo D (2018) Racial disparities in omission of oncotype DX but no racial disparities in chemotherapy receipt following completed oncotype DX test results. Breast Cancer Res Treat 168(1):207–220. https://doi.org/10.1007/s10549-017-4587-8
Parsons BM, Landercasper J, Smith AL, Go RS, Borgert AJ, Dietrich LL (2016) 21-Gene recurrence score decreases receipt of chemotherapy in ER+ early-stage breast cancer: an analysis of the NCDB 2010–2013. Breast Cancer Res Treat 159(2):315–326. https://doi.org/10.1007/s10549-016-3926-5
Jasem J, Amini A, Rabinovitch R, Borges VF, Elias A, Fisher CM, Kabos P (2016) 21-Gene recurrence score assay as a predictor of adjuvant chemotherapy administration for early-stage breast cancer: an analysis of use, therapeutic implications, and disparity profile. J Clin Oncol 34(17):1995–2002. https://doi.org/10.1200/jco.2015.65.0887
Bhutiani N, Egger ME, Ajkay N, Scoggins CR, Martin RC 2nd, McMasters KM (2018) Multigene signature panels and breast cancer therapy: patterns of use and impact on clinical decision making. J Am Coll Surg 226(4):406–412.e401. https://doi.org/10.1016/j.jamcollsurg.2017.12.043
Ricks-Santi LJ, McDonald JT (2017) Low utility of Oncotype DX(R) in the clinic. Cancer Med 6(3):501–507. https://doi.org/10.1002/cam4.837
Roberts MC, Weinberger M, Dusetzina SB, Dinan MA, Reeder-Hayes KE, Carey LA, Troester MA, Wheeler SB (2016) Racial variation in the uptake of Oncotype DX testing for early-stage breast cancer. J Clin Oncol 34(2):130–138. https://doi.org/10.1200/jco.2015.63.2489
Trosman JR, Van Bebber SL, Phillips KA (2010) Coverage policy development for personalized medicine: private payer perspectives on developing policy for the 21-gene assay. J Oncol Pract 6(5):238–242. https://doi.org/10.1200/jop.000075
Genomic Health (2019) https://www.oncotypeiq.com/en-US/breast-cancer/patients-and-caregivers/stage-0-dcis/insurance-coverage-and-financial-assistance. Accessed 15 Oct 2019
Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL (2016) Comparison of SEER treatment data with medicare claims. Med Care 54(9):e55–64. https://doi.org/10.1097/mlr.0000000000000073
Carlson JJ, Roth JA (2013) The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat 141(1):13–22. https://doi.org/10.1007/s10549-013-2666-z
Kurian AW, Bondarenko I, Jagsi R, Friese CR, McLeod MC, Hawley ST, Hamilton AS, Ward KC, Hofer TP, Katz SJ (2018) Recent trends in chemotherapy use and oncologists' treatment recommendations for early-stage breast cancer. J Natl Cancer Inst 110(5):493–500. https://doi.org/10.1093/jnci/djx239
Funding
This study was supported by National Cancer Institute Surveillance, Epidemiology, and End Results program (NCI SEER HHSN261201300016I), the Centers for Disease Control and Prevention National Program of Cancer Registries (CDC NPCR U58DP003915), National Institutes of Health/National Institute on Minority Health and Health Disparities (NIH/NIMHD R15MD012387), and Clemson University internal funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2020_5557_MOESM2_ESM.tif
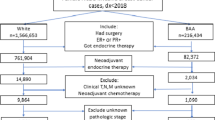
Supplementary file2 Supplemental figure 1. Flow chart for patient selection. A. Patient selection for objective 1; B. Patient selection for objective 2; C. Patient selection for objective 3. (TIF 9914 kb)
10549_2020_5557_MOESM3_ESM.tif
Supplementary file3 Supplemental figure 2. Adjusted odds ratio of receiving Oncotype DX test for race/ethnicity (non-Hispanic white patients as reference group), female hormone receptor positive breast cancer patients, 17 SEER registries, 2004-2015. A. Non-Hispanic black patients with negative axillary lymph node; B. Non-Hispanic Asian or Pacific Islander patients with negative axillary lymph node; C. Hispanic patients with negative axillary lymph node; D. Non-Hispanic black patients with 1-3 positive axillary lymph nodes; E. Non-Hispanic Asian or Pacific Islander patients with 1-3 positive axillary lymph nodes; F. Hispanic patients with 1-3 positive axillary lymph nodes. (TIF 130 kb)
10549_2020_5557_MOESM4_ESM.tif
Supplementary file4 Supplemental figure 3. Kaplan-Meier plot of breast cancer-specific survival and overall survival by Oncotype DX test order status, stratified by lymph node status, tumor size, and tumor grade, 17 SEER registries, 2004-2015. A. Negative axillary lymph node, tumor size 0.6-2.0cm, well differentiated; B. Negative axillary lymph node, tumor size 0.6-2.0cm, moderately differentiated; C. Negative axillary lymph node, tumor size 0.6-2.0cm, poorly differentiated/undifferentiated; D. Negative axillary lymph node, tumor size 2.1-5.0cm, well differentiated; E. Negative axillary lymph node, tumor size 2.1-5.0cm, moderately differentiated; F. Negative axillary lymph node, tumor size 2.1-5.0cm, poorly differentiated/undifferentiated; G. 1-3 positive axillary lymph node, tumor size ≤2.0cm, well differentiated; H. 1-3 positive axillary lymph node, tumor size ≤2.0cm, moderately differentiated; I. 1-3 positive axillary lymph node, tumor size ≤2.0cm, poorly differentiated/undifferentiated; J. 1-3 positive axillary lymph node, tumor size 2.1-5.0cm, well differentiated; K. 1-3 positive axillary lymph node, tumor size 2.1-5.0cm, moderately differentiated; L. 1-3 positive axillary lymph node, tumor size 2.1-5.0cm, poorly differentiated/undifferentiated. (TIF 717 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Hsieh, MC., Petkov, V. et al. Trend and survival benefit of Oncotype DX use among female hormone receptor-positive breast cancer patients in 17 SEER registries, 2004–2015. Breast Cancer Res Treat 180, 491–501 (2020). https://doi.org/10.1007/s10549-020-05557-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05557-x