Abstract
Background
For patients with hormone receptor (HR)-positive advanced breast cancer, whether the combination of anastrozole and fulvestrant is more effective than anastrozole alone is controversial. Our meta-analysis aimed to compare the efficacy and safety of the two therapies.
Methods
We retrieved relevant studies in Embase, the Cochrane Library, Ovid MEDLINE, PubMed, ScienceDirect, Web of Science, Scopus, and Google Scholar. The primary outcomes were overall survival (OS) and progression-free survival (PFS). The secondary outcomes were the disease control rate (DCR), the objective response rate (ORR), and adverse events (AEs).
Results
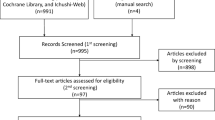
Five articles based on 4 randomized controlled trials containing 2146 patients were identified in our meta-analysis. The combination group had better efficacy in the endpoints of OS (hazard ratio [HR] 0.86; 95% confidence interval [CI] 0.74–0.99, p = 0.03) and PFS (HR 0.87; 95% CI 0.77–0.97, p = 0.02). Regarding the ORR, DCR, total AEs and grade 3–5 AEs, we found no difference between the two treatments. The combination group showed a clearly higher rate of treatment discontinuations (95% CI 1.05–3.60, p = 0.03) and AEs leading to death (95% CI 1.12–9.11, p = 0.03). The subgroup analysis of AEs showed an increased incidence of extremity or muscle pain, hematologic effects, gastrointestinal disorders, and hot flashes in the combination group.
Conclusions
For HR-positive advanced breast cancer patients, the combination of anastrozole and fulvestrant appears to be superior to anastrozole alone in extending PFS and OS, despite relatively serious AEs.





Similar content being viewed by others
Abbreviations
- AI:
-
Aromatase inhibitor
- HR-positive:
-
Hormone receptor positive
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- OSR:
-
Overall survival rate
- PFSR:
-
Progression-free survival rate
- ORR:
-
Objective response rate
- DCR:
-
Disease control rate
- AEs:
-
Adverse effects
- CI:
-
Confidence interval
- A:
-
Anastrozole
- F:
-
Fulvestrant
- PRISMA:
-
Preferred reporting items for systematic review and meta-analysis
- HR:
-
Hazard ratios
- RR:
-
Risk ratio
- RCT:
-
Randomized controlled trial
- GRADE:
-
Grading of recommendations assessment, development, and evaluation
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Harbeck N, Gnant M (2017) Breast cancer. The Lancet 389(10074):1134–1150
Early Breast Cancer Trialists Collaborative Group (EBCTCG) (2015) Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. The Lancet 386(10001):1341–1352
Van Hellemond IEG, Vriens IJH, Peer PGM, Swinkels ACP, Smorenburg CH, Seynaeve CM et al (2019) Efficacy of anastrozole after tamoxifen in early breast cancer patients with chemotherapy-induced ovarian function failure. Int J Cancer 145(1):274–283
Hanamura T, Hayashi S (2017) Overcoming aromatase inhibitor resistance in breast cancer: possible mechanisms and clinical applications. Breast Cancer 25(4):379–391
Blackburn SA, Parks RM, Cheung K-L (2018) Fulvestrant for the treatment of advanced breast cancer. Expert Rev Anticancer Ther 18(7):619–628
Shafaee MN, Ellis MJ (2018) Fulvestrant in management of hormone receptor-positive metastatic breast cancer. Future Oncol 14(18):1789–1800
Lee CI, Goodwin A, Wilcken N (2017) Fulvestrant for hormone-sensitive metastatic breast cancer. Cochrane Database Syst Rev 1:CD011093
El Sayed R, El Jamal L, El Iskandarani S, Kort J, Abdel Salam M, Assi H (2019) Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: insights to sequencing treatment and overcoming resistance based on clinical trials. Front Oncol 9:510
Nathan MR, Schmid P (2017) A review of fulvestrant in breast cancer. Oncol Ther 5(1):17–29
Bergh J, Jonsson PE, Lidbrink EK, Trudeau M, Eiermann W, Brattström D et al (2012) FACT: an open label randomized phase III study of fulvestrant and anastrozole in combination compared with anastrozole alone as first-line therapy for patients with receptor-positive postmenopausal breast cancer. J Clin Oncol 30:1919–1925
Robertson JF, Dixon JM, Sibbering DM, Jahan A, Ellis IO, Channon E et al (2013) A randomized trial to assess the biological activity of short-term (pre-surgical) fulvestrant 500 mg plus anastrozole versus fulvestrant 500 mg alone or anastrozole alone on primary breast cancer. Breast Cancer Res 15(2):R18
Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR et al (2012) Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med 367:435–444
Higgins JPT, Altman DG, Gotzsche PC, Jüni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928–d5928
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Atkins D, Best D, Briss P, Eccles M, Falck-Ytter Y, Flottorp S, GRADE Working Group (2004) Grading quality of evidence and strength of recommendations. BMJ 328(7454):1490
Egger M, Davey SG, Schneider M, Minde C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Mehta RS, Barlow WE, Albain KS, Vandenberg TA, Dakhil SR, Tirumali NR et al (2019) Overall survival with fulvestrant plus anastrozole in metastatic breast cancer. N Engl J Med 380(13):1226–1234
Ruiz-Borrego M, Guerrero-Zotano A, Bermejo B, Ramos M, Cruz J, Baena-Cañada JM et al (2019) Phase III evaluating the addition of fulvestrant (F) to anastrozole (A) as adjuvant therapy in postmenopausal women with hormone receptor-positive HER2-negative (HR+/HER2-) early breast cancer (EBC): results from the GEICAM/2006-10 study. Breast Cancer Res Treat 177(1):115–125
Cardoso F, Costa A, Senkus E (2017) 3rd ESOESMO international consensus guidelines for Advanced Breast Cancer (ABC 3). Breast 31:244–259
Rugo HS, Rumble RB, Macrae E, Barton DL, Connolly HK, Dickler MN et al (2016) Endocrine therapy for hormone receptor-positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol 34:3069–3103
Reinert T, Barrios CH (2017) Overall survival and progression-free survival with endocrine therapy for hormone receptor-positive, HER2-negative advanced breast cancer: review. Ther Adv Med Oncol 9(11):693–709
Robertson JF, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A et al (2016) Fulvestrant 500 mg versus anastrozole 1 mg for hormone receptor-positive advanced breast cancer (FALCON): an international, randomised, double-blind, phase 3 trial. Lancet 388(10063):2997–3005
Hertz DL, William EB, Kelley MK, Kathy SA, Ted AV, Shaker RD et al (2016) Fulvestrant decreases anastrozole drug concentrations when taken concurrently by patients with metastatic breast cancer treated on SWOG study S0226. Br J Clin Pharmacol 81(6):1134–1141
Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, Klijn JG et al (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359:2131–2139
Johnston SR, Kilburn LS, Ellis P, Dodwell D, Cameron D, Hayward L et al (2013) Fulvestrant plus anastrozole or placebo versus exemestane alone after progression on non-steroidal aromatase inhibitors in postmenopausal patients with hormone-receptor-positive locally advanced or metastatic breast cancer (SoFEA): a composite, multicentre, phase 3 randomised trial. Lancet Oncol 14(10):989–998
De Placido S, Gallo C, De Laurentiis M, Bisagni G, Arpino G, Sarobba MG et al (2018) Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): a randomised, phase 3 trial. Lancet Oncol 19(4):474–485
Greenlee H, Crew KD, Capodice J, Awad D, Buono D, Shi Z et al (2016) Randomized sham-controlled pilot trial of weekly electro-acupuncture for the prevention of taxane-induced peripheral neuropathy in women with early stage breast cancer. Breast Cancer Res Treat 156(3):453–464
Tan PS, Haaland B, Montero AJ, Lopes G (2013) A meta-analysis of anastrozole in combination with fulvestrant in the first line treatment of hormone receptor positive advanced breast cancer. Breast Cancer Res Treat 138(3):961–965
Acknowledgements
The authors thank professor Jichun Liu, MD, PhD (The second affiliated hospital of Nanchang University) for his advice on statistics and professor Xiaoshu Cheng, MD, PhD (The second affiliated hospital of Nanchang University) for his data collection. This study was supported by National Natural Science Foundation of China (NSFC), number of grants (81560345), Natural Science Foundation of Jiangxi Province (Grant No. 20161BAB215237). The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Funding
This study was funded by National Natural Science Foundation of China (NSFC), number of Grants (81560345), Natural Science Foundation of Jiangxi Province (Grant No. 20161BAB215237).
Author information
Authors and Affiliations
Contributions
ML had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: ML and WZ. Critical revision of the manuscript for important intellectual content: ML, YX, CL, MC and FY. Statistical analysis: ML. Supervision: YW and WZ.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No human is involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10549_2020_5551_MOESM1_ESM.tif
Supplementary file1 Figure S1 Risk of bias graph and risk of bias summary judged by the Cochrane reviewer’s handbook. (TIF 2656 kb)
10549_2020_5551_MOESM2_ESM.tif
Supplementary file2 Figure S2 Forest plots of RR of OSR for five years associated with anastrozole plus fulvestrant versus anastrozole alone. (TIF 1351 kb)
10549_2020_5551_MOESM3_ESM.tif
Supplementary file3 Figure S3 Forest plots of RR of PFSR for five years associated with anastrozole plus fulvestrant versus anastrozole alone. (TIF 1710 kb)
10549_2020_5551_MOESM4_ESM.tif
Supplementary file4 Figure S4 Forest plots of RR of ORR (A) and DCR (B) associated with anastrozole plus fulvestrant versus anastrozole alone. (TIF 1476 kb)
10549_2020_5551_MOESM6_ESM.tif
Supplementary file6 Figure S6 Begg’s and Egger’s tests for comparisons of PFS (A), OS (B) and total AEs (C). (TIF 3731 kb)
Rights and permissions
About this article
Cite this article
Li, M., Xiong, Y., Liao, C. et al. Anastrozole plus fulvestrant vs. anastrozole alone for hormone receptor-positive advanced breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer Res Treat 180, 269–278 (2020). https://doi.org/10.1007/s10549-020-05551-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-020-05551-3