Abstract
Purpose
National guidelines define adequate axillary lymph node dissections as those yielding ≥ 10 lymph nodes (LNs). We aimed to identify the optimal LN yield among node-positive patients.
Methods
Using the National Cancer Data Base (2010–2015), we categorized node-positive patients as follows: (1) neoadjuvant chemotherapy (NAC, cN1–3 or ypN1mi-3) or (2) upfront surgery (pN1–3). A restricted cubic splines model was used to estimate LN retrieval thresholds associated with change in overall survival (OS).
Results
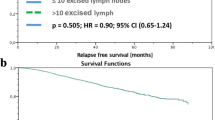
129,685 patients were identified: 21.2% NAC, 78.8% upfront surgery. Low, moderate, and high retrieval thresholds were estimated to be 1–6, 7–21, and > 21 LNs (upfront surgery), and 1–7, 8–22, and > 22 LNs (NAC). In an adjusted model, high versus low LN yield was associated with greater receipt of adjuvant chemotherapy (upfront surgery OR 1.96, p < 0.001) and greater use of adjuvant radiation (upfront surgery OR 1.08, p = 0.02; NAC OR 1.23, p = 0.002). After adjustment, high versus low LN retrieval was associated with improved OS (upfront surgery HR 0.86, p < 0.001; NAC HR 0.77, p < 0.001). Worse OS was associated with retrieving fewer LNs, likely as a result of an under-staged axilla and missed opportunity for adjuvant therapy, while better OS was independently associated with retrieval of up to approximately 20 LNs, after which survival did not improve.
Conclusion
In node-positive breast cancer, the number of nodes retrieved is significantly associated with an increased positive nodal count and greater use of adjuvant therapy. Removal of approximately 20 LNs may improve survival by both more accurate nodal staging and increased adjuvant therapy use.


Similar content being viewed by others
References
Giuliano AE, Hunt KK, Ballman KV et al (2011) Axillary dissection vs. no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 305:569–575
National Comprehensive Cancer Network (2018) NCCN, Invasive Cancer, Surgical Axillary Staging. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 4 Jan 2019
Luini A, Gatti G, Ballardini B et al (2005) Development of axillary surgery in breast cancer. Ann Oncol 16:259–262
Axelsson CK, Mouridsen HT, Zedeler K (1992) Axillary dissection of level I and II lymph nodes is important in breast cancer classification. The Danish Breast Cancer Cooperative Group (DBCG). Eur J Cancer 28A:1415–1418
Kiricuta CI, Tausch J (1992) A mathematical model of axillary lymph node involvement based on 1446 complete axillary dissections in patients with breast carcinoma. Cancer 69:2496–2501
Veronesi U, Luini A, Galimberti V, Marchini S, Sacchini V, Rilke F (1990) Extent of metastatic axillary involvement in 1446 cases of breast cancer. Eur J Surg Oncol 16:127–133
Desquilbet L, Mariotti F (2010) Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 29:1037–1057
Harrell FE (2015) Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer International Publishing, New York
Akaike H (1998) Information theory and an extension of the maximum likelihood principle. Selected Papers of Hirotogu Akaike. Springer, New York, NY, pp 199–213
Gradishar WJ, Anderson BO, Aft R, et al (2018) National Comprehensive Cancer Network. NCCN, Invasive Cancer, Version 1.2018. In Kumar R, Shead DA (eds)
Caudle AS, Yang WT, Krishnamurthy S et al (2016) Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of target axillary dissection. J Clin Oncol 34:1072–1078
Kuehn T, Bauerfeind I, Fehm T et al (2013) Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 14:609–618
Boughey JC, Suman VJ, Mittendorf EA et al (2013) Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (alliance) clinical trial. JAMA 310:1455–1461
Nemoto T, Vana J, Bedwani RN et al (1980) Management and survival of female breast cancer: results of a National Survey by the American College of Surgeons. Cancer 45:2917–2924
Carter CL, Allen C, Henson DE (1989) Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 63:181–187
Plichta JK, Campbell BM, Mittendorf EA et al (2018) Anatomy and breast cancer staging: is it still relevant? Surg Oncol Clin N Am 27:51–67
AJCC Cancer Staging Manual (ed 8th) (2016) Springer International Publishing, New York
Chen S, Liu Y, Huang L et al (2014) Lymph node counts and ratio in axillary dissections following neoadjuvant chemotherapy for breast cancer: a better alternative to traditional pN staging. Ann Surg Oncol 21:42–50
Whelan TJ, Olivotto IA, Parulekar WR et al (2015) Regional nodal irradiation in early-stage breast cancer. N Engl J Med 373:307–316
Poortmans PM, Collette S, Kirkove C et al (2015) Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 373:317–327
Overgaard M, Nielsen HM, Overgaard J (2007) Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol 82:247–253
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C et al (2014) Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 383:2127–2135
Olivotto IA, Bajdik CD, Ravdin PM et al (2005) Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol 23:2716–2725
Wishart GC, Azzato EM, Greenberg DC et al (2010) PREDICT: a New UK prognostic model that predicts survival following surgery for invasive breast cancer. Breast Cancer Res 12:R1
Kuru B (2006) Prognostic significance of total number of nodes removed, negative nodes removed, and ratio of positive nodes to removed nodes in node positive breast carcinoma. Eur J Surg Oncol 32(10):1082–1088
Wang X, Ji C, Chi H et al (2018) How many ELNs are optimal for breast cancer patients with more than three PLNs who underwent MRM? A large population-based study. Onco Targets Ther 11:1005–1011
Sormani MP (2009) The Will Rogers phenomenon: the effect of different diagnostic criteria. J Neurol Sci 287(Suppl 1):S46
Mallin K, Palis BE, Watroba N et al (2013) Completeness of American Cancer Registry Treatment Data: implications for Quality of Care Research. J Am Coll Surg 216:428–437
Funding
Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 1KL2TR002554 (PI: Svetkey). Dr. R. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH Grant P30CA014236 (PI: Kastan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Due to use of national de-identified data (National Cancer Data Base, NCDB), our institutional review board granted the study exempt status and no individual informed consent was needed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rosenberger, L.H., Ren, Y., Thomas, S.M. et al. Axillary lymph node dissection in node-positive breast cancer: are ten nodes adequate and when is enough, enough?. Breast Cancer Res Treat 179, 661–670 (2020). https://doi.org/10.1007/s10549-019-05500-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-019-05500-9